Extractive optimization of antioxidants and phenolic compounds from Anacyclus pyrethrum
DOI:
https://doi.org/10.55779/nsb15411616Keywords:
Anacyclus pyrethrum, antioxidant activity, polyphenols, solvent extraction, surface design methodologyAbstract
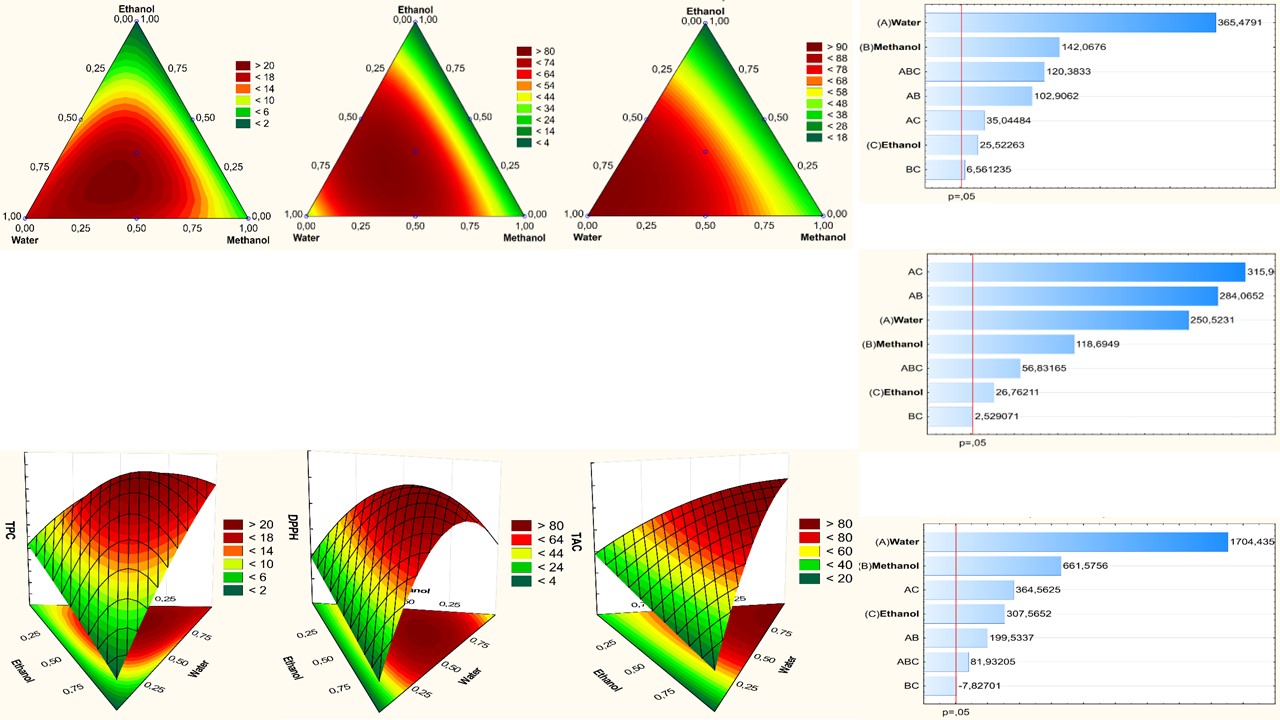
Anacyclus pyrethrum (L.) Lag. is a Moroccan endemic species well appreciated as a remedy against toothache, digestive disorders, and as a tonic agent for the nervous system. This work aims to select the best solvents for extracting antioxidants and optimize their extraction using a surface mixture design. In this study, eleven solvents with different polarities were screened for their efficiency to extract total phenolic compounds and other molecules endowed with antioxidant activity. The antioxidant activity was measured using 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging and total antioxidant activities. The selected solvent was subjected to response surface methodology using a simplex axial mixture design to optimize the extraction of polyphenols and antioxidants. The results showed a significant influence of solvent nature on extraction. Water, ethanol, chloroform, and methanol were the most effective solvents to achieve good polyphenol yields. The best yield is obtained using the tertiary mixture “water-methanol-ethanol”. The anti-radical activity in A. pyrethrum was significantly influenced by the extraction solvent's nature and the mixture's nature. The percentage of inhibition of DPPH was higher in both extracts obtained with the ternary mixture and binary mixtures “water-methanol” and “water-ethanol”. the best total antioxidant capacity was observed for pure water, followed by binary mixtures including water. This study revealed a good synergetic effect between water and both ethanol and methanol on extraction efficiency. Furthermore, the ternary mixture with the following proportions: water 75%; ethanol 11%; methanol 14% was the most efficient.
Metrics
References
Aazza S (2021). Application of multivariate optimization for phenolic compounds and antioxidants extraction from Moroccan Cannabis sativa waste. Journal of Chemistry 1-11. https://doi.org/10.1155/2021/9738656
Amine D, Mohamed B, Jamal I, Laila N (2017). Antibacterial activity of aqueous extracts of Anacyclus pyrethrum (L) Link and Corrigiola telephiifolia Pourr. from the Middle Atlas region, Morocco. European Scientific Journal 13(33):116. https://doi.org/10.19044/esj.2017.v13n33p116
Bouaziz M, Dhouib A, Loukil S, Boukhris M, Sayadi S (2009). Polyphenols content, antioxidant and antimicrobial activities of extracts of some wild plants collected from the south of Tunisia. African Journal of Biotechnology 8(24):7017-7027. https://doi.org/10.5897/AJB2009.000-9545
Brand-Williams W, Cuvelier ME, Berset C (1995). Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology 28(1):25-30. https://doi.org/10.1016/S0023-6438(95)80008-5
Chemat F, Rombaut N, Sicaire A-G, Meullemiestre A, Fabiano-Tixier A-S, Abert-Vian M (2017). Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrasonics Sonochemistry 34:540-560. https://doi.org/10.1016/j.ultsonch.2016.06.035
De Lima Da Silva N, Batistella ĆB, Filho RM, Maciel MRW (2009). Biodiesel production from castor oil: Optimization of alkaline ethanolysis. Energy and Fuels 23(11):5636-5642. https://doi.org/10.1021/ef900403j
DiCiaulaa MC, Lopesa GC, Scarminiob IS, Melloa JCP de (2014). Optimization of solvent mixtures for extraction from bark of Schinus terebinthifolius by a statistical mixture-design technique and development of a uv-vis spectrophotometric method for analysis of total polyphenols in the extract. Quim. Nova 37(1):158-163.
El Alami A (2022). Biodiversity loss in the Moroccan central High Atlas, its impact on local ecosystems and national economy, and wildlife conservation strategy: Findings from 20 years of research. Journal of Analytical Sciences and Applied Biotechnology 4(2):81-96. https://doi.org/10.48402/IMIST.PRSM/jasab-v4i2.35045
Elazzouzi H, Fadili K, Cherrat A, Amalich S, Zekri N, Zerkani H, Tagnaout I, Hano C, Lorenzo JM, Zair T (2022). Phytochemistry, biological and pharmacological activities of the Anacyclus pyrethrum (L.) Lag: A systematic review. Plants 11(19):2578. https://doi.org/10.3390/plants11192578
Fennane M, Rejdali M (2016). Aromatic and medicinal plants of Morocco: Richness, diversity and threats. Bulletin de l’institut Scientifiue, Rabat, Section Sciences de La Vie 38:27-42.
Ghafoor K, Choi YH, Jeon JY, Jo IH (2009). Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. Journal of Agricultural and Food Chemistry 57(11):4988-4994. https://doi.org/10.1021/jf9001439
Iuchi K, Hatano Y, Yagura T (2008). Heterocyclic organ bismuth (III) induces apoptosis of human promyelocytic leukemic cells through activation of caspases and mitochondrial perturbation. Biochemical Pharmacology 76(8):974-986. https://doi.org/10.1016/j.bcp.2008.07.038
Kaczorová D, Karalija E, Dahija S, Bešta-Gajević R, Parić A, Ćavar Zeljković S (2021). Influence of extraction solvent on the phenolic profile and bioactivity of two Achillea species. Molecules 26(6):1601. https://doi.org/10.3390/molecules26061601
Ksibi I El, Slama R Ben, Faidi K, Ticha M Ben, M’henni MF (2015). Mixture approach for optimizing the recovery of colored phenolics from red pepper (Capsicum annum L.) by-products as potential source of natural dye and assessment of its antimicrobial activity. Industrial Crops and Products 70:34-40. https://doi.org/10.1016/j.indcrop.2015.03.017
Ksouri R, Megdiche W, Falleh H, Trabelsi N, Boulaaba M, Smaoui A, Abdelly C (2008). Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. Comptes Rendus Biologies 331(11):865-873. https://doi.org/10.1016/j.crvi.2008.07.024
Kumar V, Choudhary AK (2016). Akarkara: a versatile medicinal plant – a review. Journal of Ayurveda and Holistic Medicine 1-14.
Libbey LM, Walradt JP (1968). 3,5-di-Tert-butyl-4-hydroxytoluene (BHT) as an artifact from diethyl ether. Lipids 3(6):561-561. https://doi.org/10.1007/BF02530903
Liyana-Pathirana CM, Shahidi F (2006). Antioxidant properties of commercial soft and hard winter wheats (Triticum aestivum L.) and their milling fractions. Journal of the Science of Food and Agriculture 86(3):477-485. https://doi.org/10.1002/jsfa.2374
Liyanapathirana C, Shahidi F (2005). Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chemistry 93(1):47-56. https://doi.org/10.1016/j.foodchem.2004.08.050
Ma T, Wang Q, Wu H (2010). Optimization of extraction conditions for improving solubility of peanut protein concentrates by response surface methodology. LWT - Food Science and Technology 43(9):1450-1455. https://doi.org/10.1016/j.lwt.2010.03.015
Mohammedi Z, Atik F (2011). Impact of solvent extraction type on total polyphenols content and biological activity from Tamarix aphylla (L.) Karst. International Journal of Pharma and Bio Sciences 2:609-615.
Montgomery DC (2012). Design and analysis of experiments Eighth Edition. Design 2:757. https://doi.org/10.1198/tech.2006.s372
Naczk M, Shahidi F (2004). Extraction and analysis of phenolics in food. Journal of Chromatography A 1054(1-2):95-111. https://doi.org/10.1016/j.chroma.2004.08.059
Nawaz H, Shi J, Mittal GS, Kakuda Y (2006). Extraction of polyphenols from grape seeds and concentration by ultrafiltration. Separation and Purification Technology 48(2):176-181. https://doi.org/10.1016/j.seppur.2005.07.006
Pandey S, Rani Kushwaha G, Singh A, Singh A (2018). Chemical composition and medicinal uses of Anacyclus pyrethrum. Pharma Science Monitor 9(1):551-560.
Prieto P, Pineda M, Aguilar M (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Analytical Biochemistry 269(2):337-341. https://doi.org/10.1006/abio.1999.4019
Sampaio CRP, Anastácio LMC, De Francisco TMG, Ribani RH (2015). Anthocyanins and phenolic compounds in five ripening stages of Byrsonima ligustrifolia after extraction optimization. Journal of Food and Nutrition Research 54(4):365-378.
Santos Felix AC, Novaes CG, Pires Rocha M, Barreto GE, do Nascimento BB, Giraldez Alvarez LD (2018). Mixture design and Doehlert matrix for the optimization of the extraction of phenolic compounds from Spondias mombin L Apple bagasse agro-industrial residues. Frontiers in Chemistry 5:1-8. https://doi.org/10.3389/fchem.2017.00116
Subasri G, John SA (2015). Screening of phytochemical compounds, trace metals and antimicrobial activity of Anacyclus pyrethrum. International Journal of Advances in Scientific Research 1(08):322-328. https://doi.org/10.7439/ijasr
Trabelsi N, Falleh H, Jallali I, Daly A Ben, Hajlaoui H, Smaoui A, Abdelly C, Ksouri R (2012). Variation of phenolic composition and biological activities in Limoniastrum monopetalum L. organs. Acta Physiologiae Plantarum 34(1):87-96. https://doi.org/10.1007/s11738-011-0807-8
Trabelsi N, Megdiche W, Ksouri R, Falleh H, Oueslati S, Soumaya B, Hajlaoui H, Abdelly C (2010). Solvent effects on phenolic contents and biological activities of the halophyte Limoniastrum monopetalum leaves. LWT - Food Science and Technology 43(4):632-639. https://doi.org/10.1016/j.lwt.2009.11.003
Turkmen N, Velioglu Y, Sari F, Polat G (2007). Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules 12(3):484-496. https://doi.org/10.3390/12030484
Venkatesan T, Choi Y-W, Kim Y-K (2019). Impact of different extraction solvents on phenolic content and antioxidant potential of Pinus densiflora Bark extract. BioMed Research International 2019:1-14. https://doi.org/10.1155/2019/3520675
Wang L, Weller CL (2006). Recent advances in extraction of nutraceuticals from plants. Trends in Food Science and Technology 17(6):300-312. https://doi.org/10.1016/j.tifs.2005.12.004
Xiao W, Han L, Shi B (2008). Microwave-assisted extraction of flavonoids from Radix Astragali. Separation and Purification Technology 62(3):614-618. https://doi.org/10.1016/j.seppur.2008.03.025
Yahyaoui M, Ghazouani N (2017). Comparison of the Effect of various extraction methods on the phytochemical composition and antioxidant activity of Thymelaea hirsuta L. aerial parts in Tunisia. Biosciences, Biotechnology Research Asia 14(3):997-1007. https://doi.org/10.13005/bbra/2534
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Oumaima CHATER, Lahsen EL GHADRAOUI, Ahmed HARRACH, Smail AAZZA

This work is licensed under a Creative Commons Attribution 4.0 International License.
Papers published in Notulae Scientia Biologicae are Open-Access, distributed under the terms and conditions of the Creative Commons Attribution License.
© Articles by the authors; licensee SMTCT, Cluj-Napoca, Romania. The journal allows the author(s) to hold the copyright/to retain publishing rights without restriction.
License:
Open Access Journal - the journal offers free, immediate, and unrestricted access to peer-reviewed research and scholarly work, due SMTCT supports to increase the visibility, accessibility and reputation of the researchers, regardless of geography and their budgets. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author.













.png)















