Micropropagation and potential of bioactive compounds of saffron (Crocus sativus L.) for nutrition and health
DOI:
https://doi.org/10.55779/nsb14311278Keywords:
biological activities, chemical compounds, Crocus sativus, in-vitro cultureAbstract
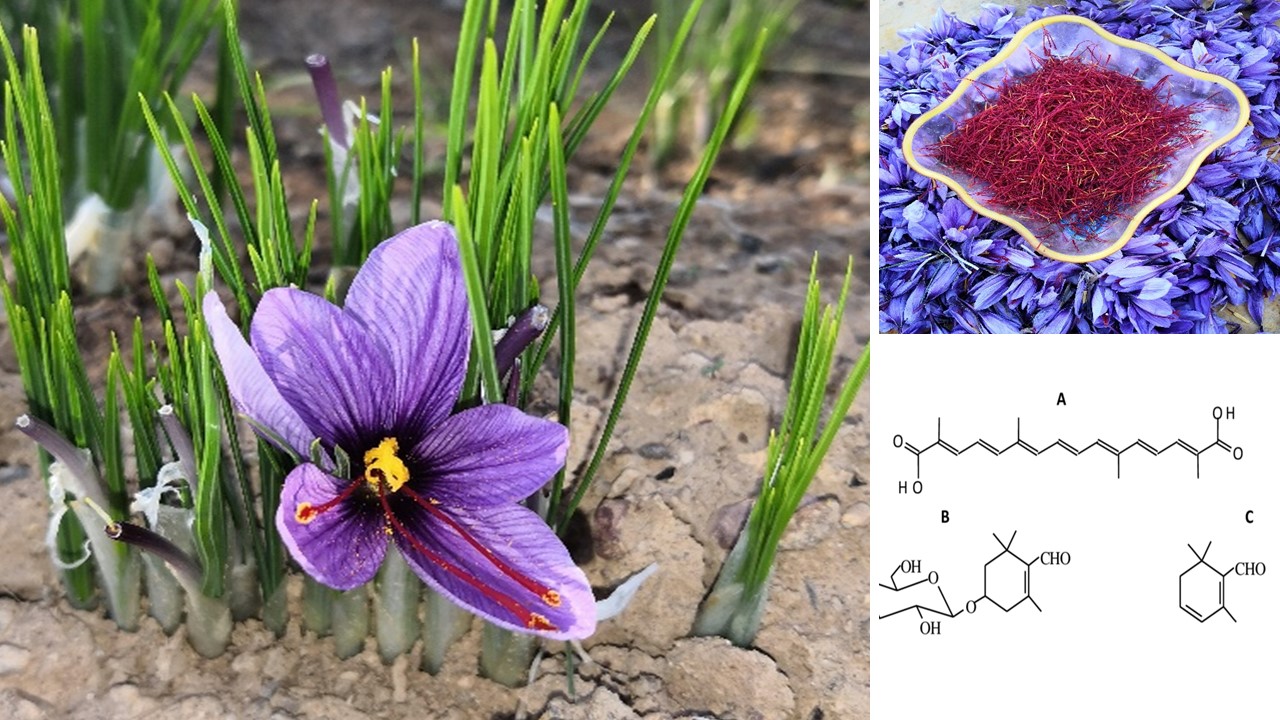
To inquire about the possibility of durable valorization of saffron, this review highlights the different techniques of in vitro culture and varietal creation of this specie. Then, a reveal main component of saffron with some pharmacological activities that make this product a serious therapeutic hope. Saffron (Crocus sativus L.) is a sterile triploid geophyte that propagated by corms. To respond to the increasing global demand for saffron, it is necessary to expand the area under cultivation. Thus, in vitro techniques can produce a large quantity of propagating material in reduced time. It is well-known that saffron is traditionally used as a coloring or flavoring agent, but recent research showed its potential for health promotion. The interest components include crocin, crocetin, picrocrocin, and safranal have all demonstrated a wide range of use in medical field. Previous studies have reported that biological activities of saffron alleviates or prevents health problems such as stomach upset, cardiovascular disease, and depression. In addition, saffron is also promising in cancer prevention due to its antioxidant properties.
Metrics
References
Abdullaev F (2007). Biological properties and medicinal use of saffron (Crocus sativus L.). Acta Horticulturae 739:339-345. https://doi.org/10.17660/ActaHortic.2007.739.44
Abdullaev FI, Espinosa-Aguirre JJ (2004). Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detection and Prevention 28(6):426-432 https://doi.org/10.1016/j.cdp.2004.09.002
Ahmadpanah M, Ramezanshams F, Ghaleiha A, Akhondzadeh S, Bahmani DS, Brand S (2019). Crocus sativus L. (saffron) versus sertraline on symptoms of depression among older people with major depressive disorders–a double-blind, randomized intervention study. Psychiatry Research 282:112613. https://doi.org/10.1016/j.psychres.2019.112613
Ahmed ABA, Rao AS, Rao MV, Taha RM (2011). Effect of picloram, additives and plant growth regulators on somatic embryogenesis of Phyla nodiflora L. Greene. Brazilian Archives of Biology and Technology 54:7-13.
Akhondzadeh S, Mostafavi SA, Keshavarz SA, Mohammadi MR, Hosseini S, Eshraghian MR (2020). A placebo controlled randomized clinical trial of Crocus sativus L. (saffron) on depression and food craving among overweight women with mild to moderate depression. Journal of Clinical Pharmacy and Therapeutics 45:134-143. https://doi.org/10.1111/jcpt.13040
Akhund-Zade IM, Muzaferova RS (1975). Studies of the effectiveness of gamma irradiation of the saffron. Radiobiology 15(2):319-322.
Alonso GL, Salinas MR, Garijo J, Sanchez-Fernadez MA (2001). Composition of crocins and picrocrocin from Spanish saffron (Crocus sativus L.). Journal of Food Quality 24:219-233. https://doi.org/10.1111/j.1745-4557.2001.tb00604.x
Amanpour A, Kelebek H, Selli S (2019). LC/HPLC methods for saffron (Crocus sativus L.). Bioactive Molecules in Food 2019:1987-2035. https://doi.org/10.1007/978-3-319-78030-6_42
Anjum N, Pal A, Tripathi YC (2015). Phytochemistry and pharmacology of saffron, the most precious natural source of colour, flavour and medicine. SMU Medical Journal 2(1):335-346.
Azadi P, Bagheri K, Gholami M, Mirmasoumi M, Moradi A, Sharafi A (2017). Thin cell layer, a suitable explant for in vitro regeneration of saffron (Crocus sativus L.). Journal of Agriculture, Science and Technology 19(6):1429-1435.
Azarabadi N, Özdemir F (2018). Determination of crocin content and volatile components in different qualities of Iranian Saffron. Gida The Journal of Food 43(3):476-489. https://doi.org/10.15237/gida.GD18018
Azgomi RND, Karimi A, Zarshenas MM, Jazani AM (2022). The mechanisms of saffron (Crocus sativus) on the inflammatory pathways of diabetes mellitus: A systematic review. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 16:102365. https://doi.org/10.1016/j.dsx.2021.102365
Azizian A, Sepaskhah AR (2014). Maize response to water, salinity and nitrogen levels: physiological growth parameters and gas exchange. International Journal of Plant Production 8(1):131-162.
Bagheri A, Vesal SR (2006). Genetics, sterility, propagation and in vitro production of secondary metabolites. In: Kafi M, Koocheki A, Rashed MH (Eds). Saffron (Crocus sativus): Production and Processing. Sci. Publishing Plymouth, England. pp 119-137.
Bakshi HA, Zoubi MSA, Faruck HL, Aljabali AA, Rabi FA, Hafiz AA, ... Tambuwala M (2020). Dietary crocin is protective in pancreatic cancer while reducing radiation-induced hepatic oxidative damage. Nutrients 12:1901. https://doi.org/10.3390/nu12061901
Bathaie SZ, Mousavi SZ (2010). New applications and mechanisms of action of saffron and its important ingredients. Critical Reviews in Food Science and Nutrition 50(8):761-786. https://doi.org/10.1080/10408390902773003
Bayrami G, Boskabady MH (2012). The potential effect of the extract of Crocus sativus and safranal on the total and differential white blood cells of ovalbumin-sensitized guinea pigs. Research in Pharmaceutical Sciences 7(4):249-255.
Bhagyalakshmi N (1999). Factors influencing direct shoot regeneration from ovary explants of saffron. Plant Cell, Tissue and Organ Culture 58(3):205-211. https://doi.org/10.1023/A:1006398205936
Bhat ZA, Kumar V, Kumar D, Khan NA, Chashoo IA, Shah MY (2012). Evaluation of anti-inflammatory potential of petal extracts Crocus sativus “Cashmerinus”. International Journal of Phytopharmacology 3(1):27-31.
Blazquez S, Olmos E, Hernández JA Hellın E, Fernández JA, Piqueras A (2009) Somatic embryogenesis in saffron (Crocus sativus L.). Histological differentiation and implication of some components of the anti-oxidant enzymatic system. Plant Cell, Tissue and Organ Culture 97(1):49-57. https://doi.org/10.1007/s11240-009-9497-y
Blázquez S, Piqueras A, Rubio C, Fernandez JA (2007). Comparative effects of BAP and TDZ on multiplication of micropropagated saffron (Crocus sativus) corms. In: Sheibani M, Azghandi AV, Nemati SH (Eds). Pakistan Journal of Biological Sciences 10(20):3564-3570.
Bononi M, Tateo F, Scaglia B, Quaglia G (2020). δ13C data of the total water-soluble fraction and triacylglycerols as related indexes for differentiating the geographical origin of saffron (Crocus sativus L.). Food Chemistry 315:1-6. https://doi.org/10.1016/j.foodchem.2020.126292.
Bukhari SI, Manzoor M, Dhar MK (2018). A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids. Biomedicine & Pharmacotherapy 98:733-745. https://doi.org/10.1016/j.biopha.2017.12.090
Byrami G, Boskabady MH, Jalali S, Farkhondeh T (2013). The effect of the extract of Crocus sativus on tracheal responsiveness and plasma levels of IL-4, IFN-γ, total NO and nitrite in ovalbumin sensitized guinea-pigs. Journal of Ethnopharmacology 147(2):530-535. https://doi.org/10.1016/j.jep.2013.03.014
Calixto JB, Beirith A, Ferreira J Santos AR, Filho Cechinel V, Yunus RA (2000). Naturally occurring antinociceptive substances from plants. Phytotherapy Research 14(6):401-418. https://doi.org/10.1002/1099-1573(200009)14:6<401::AID-PTR762>3.0.CO;2-H
Çavusoglu A, Erkel EI, Sülüsoglu M (2009). Saffron (Crocus sativus L.) studies with two mother corm dimensions on yield and harvest period under greenhouse condition. American-Eurasian Journal of Sustainable Agriculture 3(2):126-129.
Cerd´a-Bernad D, Valero-Cases E, Pastor JJ, Frutos MJ (2020). Saffron bioactives crocin, crocetin and safranal: effect on oxidative stress and mechanisms of action. Critical Reviews in Food Science and Nutrition 2020:1-18. https://doi.org/10.1080/10408398.2020.1864279
Ceriello A, Prattichizzo F, Phillip M, Hirsch IB, Mathieu C, Battelino T (2022). Glycaemic management in diabetes: old and new approaches. Lancet Diabetes Endocrinol 10(1):75e84. https://doi.org/10.1016/S2213-8587(21)00245-X
Chahota RK, Dhiman KC, Rana SS, Mohar S (2003). Efficacy of different propagating methods for higher daughter corm production in saffron (Crocus sativus L.). Indian Perfumer 47(2):155-158.
Chaloushi B, Zarghami R, Abd-Mishani C, Omidi M, Agayev YM, Pakdaman Sardood B, Pakdaman Sardood B (2007). Effects of different hormonal treatments on the callus production and plantlet regeneration in saffron (Crocus sativus L.). Pakistan Journal of Biological Sciences 10(10):1625-1631. https://doi.org/10.3923/pjbs.2007.1625.1631
Chatterjee S, Datta RN, Bhattacharyya D, Bandopadhyay SK (2005). Emollient and antipruritic effect of Itch cream in dermatological disorders: A randomized controlled trial. Research Letter 37(4):253-254.
Chen S, Wang X, Zhao B Yuan X, Wang Y, (2003). Production of crocin using Crocus sativus callus by two-stage culture system. Biotechnology Letters 25(15):1235-1238. https://doi.org/10.1023/A:1025036729160
Chichiricc`o G, Ferrante C, Menghini L, Recinella L, Leone S, Chiavaroli A, … Orlando G (2019). Crocus sativus by-products as sources of bioactive extracts: pharmacological and toxicological focus on anthers. Food and Chemical Toxicology 126:7-14. https://doi.org/10.1016/j.fct.2019.01.040
Chopra V, Flanders SA, O’Malley M (2020). Sixty-day outcomes among patients hospitalized with COVID-19. Annals of Internal Medicine 174(4):576-578. https://doi.org/10.7326/M20-5661
Chryssanthi DG, Lamari FN, Iatrou G Pylara A, Karamanos NK, Cordopatis P (2007). Inhibition of breast cancer cell proliferation by style constituents of different Crocus species. Anticancer Research 27(1):357-362. https://doi.org/10.1007/s12161-019-01610-8
D’Archivio AA, Di Vacri ML, Ferrante M Maggi MN, Nisi S, Ruggieri F (2019). Geographical discrimination of saffron (Crocus sativus L.) using ICP-MS elemental data and class modeling of PDO Zafferano dell’Aquila produced in Abruzzo (Italy). Food Analytical Methods 12(1):2572-2581. https://doi.org/10.1007/s12161-019-01610-8
Darvishi E, Zarghami R, Mishani CA, Omidi M (2007). Effects of different hormone treatments on nonembryogenic and embryogenic callus induction and time-term enzyme treatments on number and viability of isolated protoplasts in saffron (Crocus sativus L.). Acta Horticulturae 739:279-284. https://doi.org/10.17660/ActaHortic.2007.739.35
Das I, Chakrabarty RN, Das S (2004). Saffron can prevent chemically induced skin carcinogenesis in swiss albino mice. Asian Pacific Journal of Cancer Prevention 5 (1): 70-76. https://doi.org/10.1207/S15327914NC421_12
Devi K, Sharma M, Ahuja PS (2014). Direct somatic embryogenesis with high frequency plantlet regeneration and successive cormlet production in saffron (Crocus sativus L.). South African Journal of Botany 93:207-216. https://doi.org/10.1016/j.sajb.2014.04.006
Dhar A, Mehta S, Dhar G, Dhar K, Banerjee S, Van Veldhuizen P (2009). Crocetin inhibits pancreatic cancer cell proliferation and tumor progression in a xenograft mouse model. Molecular Cancer Therapy 8(2):315-323. https://doi.org/10.1158/1535-7163.MCT-08-0762
Dhar AK, Sapru R, Rekha K (1988). Studies on saffron in Kashmir: Variation in natural population and its cytological behavior. Journal of Crop Improvement 15(1):48-52.
Dıaz-Vivancos P, Majourhat K, Fernandez JA Hernandez JA, Piqueras A (2011). Study of the antioxidant enzymatic system during shoot development from cultured intercalar meristems of saffron. Plant Growth Regulation 65(1):119-126. https://doi.org/10.1007/s10725-011-9581-2
Ding BZ, Bai SH, Wu Y, Fan XP (1981). Induction of callus and regeneration of plantlets from corm of Crocus sativus L. Acta Botanica Sinica 23:119-120.
Donini P, Sonnino A (1998). Induced mutation in plant breeding: current status and future outlook. In: Jain SM, Brar DS, Ahloowalia BS (Eds). Somaclonal Variation and Induced Mutations in Crop Improvement. Current Plant Science and Biotechnology in Agriculture 32:255-291.
Dris R, Jain SM (2004). Production practices and quality assessment of food crops. Ed., Preharvest Practice, Springer. 1.
Ebrahimi F, Sahebkar A, Aryaeian N, Pahlavani N, Fallah S, Moradi N, Abbasi D, Fatemeh HA (2019). Effects of saffron supplementation on inflammation and metabolic responses in type 2 diabetic patients: a randomized, double-blind, placebo-controlled trial. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 12:2107. https://doi.org/10.2147/DMSO.S216666.
Ebrahimzadeh H, Karamian R, Noori-Daloii MR (2000) Somatic embryogenesis and regeneration of plantlet in saffron Crocus sativus L. Journal of Sciences, Islamic Republic of Iran 11:169-173.
Esmaeili N, Ebrahimzadeh H, Abdi K, Safarian S (2011). Determination of some phenolic compounds in Crocus sativus L. corms and its antioxidant activities study. Pharmacognosy Magazine 7(2011):74-80. https://doi.org/10.4103/0973-1296.75906
Fahim NK, Janati SF, Feizy J (2012). Chemical composition of agriproduct saffron (Crocus sativus L.) petals and its considerations as animal feed. GIDA 37(4):197-201.
Fatehi M, Rashidabady T, Fatehi-Hassanabad Z (2003). Effects of Crocus sativus petals’ extract on rat blood pressure and on response induced by electrical field stimulation in the rat isolated vas deferens and guinea-pig ileum. Journal of Ethnopharmacology 84(2-3):199-203. https://doi.org/10.1016/s0378-8741(02)00299-4
Feizy J, Reyhani N (2016). Gas chromatographic determination of phytosterols and fatty acids profile in saffron petals. Canadian Chemical Transactions 4(3):389-397. https://doi.org/10.13179/canchemtrans.2016.03.0329
Fernández JA (2004). Biology, biotechnology and biomedicine of saffron. In: Pandalai SG (Ed). Recent vol 2, Recent Research Developments in Plant Science, Research Signpost, Trivandrum pp 127-159.
Florindo HF, Kleiner R, Vaskovich-Koubi D (2020). Immune-mediated approaches against COVID-19. Nature Nanotechnology 15:630-645. https://doi.org/10.1038/s41565-020-0732-3
Francis D, Sorrell DA (2001). The interface between the cell cycle and plant growth regulators: a mini review. Plant Growth Regulation 33(1):1-12. https://doi.org/10.1023/A:1010762111585
Gadd CJ (1971). The dynasty of Agade and the Gutian invasion. In: Edwards ES, Gadd CJ, Hammond NGL (Eds). Cambridge Ancient History 1:417-463.
Gantait S, Vahedi M (2015). In vitro regeneration of high value spice Crocus sativus L.: A concise appraisal. Journal of Applied Research on Medicinal and Aromatic Plants 2(4):124-133. https://doi.org/10.1016/j.jarmap.2015.07.003
George L, Eapen S (1993). Influence of genotype and explants source on somatic embryogenesis in peanut. Oleagineux 48(9):361-364.
George PS, Visvanath S, Ravishankar GA, Venkataraman LV (1992.) Tissue culture of saffron (Crocus sativus L.): Somatic embryogenesis and shoot regeneration. Food Biotechnology 6(3):217-223. https://doi.org/10.1080/08905439209549835
Ghoreshiand SG, Moodi S (2015). Assessment of nitrogen application form impacts on Saffron (Crocus sativus L.) productivity under salt stress. Journal of Applied Environmental and Biological Sciences 5 (21S):667-674.
Gresta F, Lombardo GM, Siracusa L, Ruberto G (2008) Saffron. an alternative crop for sustainable agricultural systems. A review. Agronomy for Sustainable Development 28(1):95-112. https://doi.org/10.1051/agro:2007030
Gui YL, Xu TY, Gu SR, Liu SQ, Sun GD, Zhang Q (1998). Corm formation of saffron crocus in vitro. Acta Botanica Sinica 30:338-340.
Hartwell JL (1982). Plants used against cancer: A survey. Lawrence MA (Ed). Quarterman Publications, 34:204-255.
Harvanaghi ES, Arkun G (2018). Investigating the chemical composition of saffron (Crocus sativus) growing in different geographic regions. Asian Journal of Agriculture and Food Sciences 6(1):1-6.
Hashemi P, Erim FB (2019). Analysis of Vitamin B2 in saffron stigmas (Crocus sativus L) by capillary electrophoresis coupled with laser-induced fluorescence detector. Food Analytical Methods 9(8):2395-2399. https://doi.org/10.1007/s12161-016-0430-9
Homes J, Legros M, Jaziri M (1987). In vitro multiplication of Crocus sativus L. Acta Horticulturae 11(212):675-676.
Hossain Z, Mustafa G, Komatsu S (2015). Plant responses to nanoparticle stress. International Journal of Molecular Sciences 16(11):26644-26653. https://doi.org/10.3390/ijms161125980
Hosseinzadeh H, Ramezani M, Salmani GA (2000). Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. Journal of Ethnopharmacology 73:379-385. https://doi.org/10.1186/1471-2210-2-7
Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, ... Cao B (2021). 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet 39:220-232. https://doi.org/10.1016/S0140-6736(20)32656-8
Husaini AM, Jan KN, Wani GA (2021). Saffron: A potential drug-supplement for severe acute respiratory syndrome coronavirus (COVID) management Heliyon 7(5):e07068. https://doi.org/10.1016/j.heliyon.2021.e07068
Jadouali SM, Atifi H, Mamouni R Majourhat K, Bouzoubaâ Z, Laknifli A, Faouzi A (2018) Chemical characterization and antioxidant compounds of flower parts of Moroccan Crocus sativus L. Journal of the Saudi Society of Agricultural Sciences 18 (4):476-480. https://doi.org/10.1016/j.jssas.2018.03.007
Jessie SW, Krishnakantha TP (2005). Inhibition of human platelet aggregation and membrane lipid peroxidation by food spice, saffron. Molecular and Cellular Biochemistry 278(1-2):59-63. https://doi.org/10.1007/s11010-005-5155-9
Jun Z, Xiaobin C, Fang C (2007). Factors influencing in vitro flowering from styles of saffron. Acta Horticulturae 739:313-320. https://doi.org/10.1007/s11010-005-5155-9
Karabagias IK, Koutsoumpou M, Liakou V, Kontakos S, Kontominas MG (2017). Characterization and geographical discrimination of saffron from Greece, Spain, Iran, and Morocco based on volatile and bioactivity markers, using chemometrics. European Food Research and Technology 243(9):1577-1591. https://doi.org/10.1007/s00217-017-2866-6
Karamian R (2004). Plantlet regeneration via somatic embryogenesis in four species of Crocus. Acta Horticulturae 650:253-259. https://doi.org/10.17660/ActaHortic.2004.650.28
Karaoğlu C, Çöcü S, Ipek A, Parmaksız I Uranbey S, Sarıhan E (2007). In vitro micropropagation of saffron. Acta Horticulturae 739:223-227. https://doi.org/10.17660/ActaHortic.2007.739.28
Karimi E, Oskoueian E, Hendra R, Jaafar HZ (2010). Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules 15(9):6244-6256. https://doi.org/10.3390/molecules15096244
Kashtwari M, Aijaz A, Wani AA (2018). Development of an efficient in vitro mutagenesis protocol for genetic improvement of saffron (Crocus sativus L.). Physiology and Molecular Biology of Plants 24(5):951-962. https://doi.org/10.1007/s12298-018-0576-6
Khan MA, Nagoo S, Naseer S, Nehvi FA, Zargar SM (2011). Induced mutation as a tool for improving corm multiplication in saffron (Crocus sativus L.). Journal of Phytology 3(7):08-10.
Khan S, Al-Qurainy F, Anwar F (2009). Sodium Azide: A chemical mutagen for enhancement of agronomic traits of crop plants. International Journal of Environmental Science and Technology 4(2009):1-21.
Khazaei KM, Jafari S, Ghorbani M, Kakhki AH, Sarfarazi M (2001). Optimization of anthocyanin extraction from saffron petals with response surface methodology. Food Analytical Methods 9(7):1993-2001. https://doi.org/10.1007/s12161-015-0375-4
Koocheki A, Ganjeali A, Abbassi F (2007). The effect of duration of incubation and photoperiod on corm and shoot characteristics of saffron plant (Crocus sativus L.). Acta Horticulturae 73:961-70. https://doi.org/10.17660/ActaHortic.2007.739.6
Koocheki A, Sayyedi MS (2016). Effects of different water supply and corn planting density on crocin, picrocrocin and safranal, nitrogen uptake and water use efficiency of saffron grown in semi-arid region. Notulae Scientia Biologicae 8(3):334-341. https://doi.org/10.15835/nsb839855
Koocheki A, Seyyedi SM, Gharaei S (2016). Evaluation of the effects of saffron-cumin intercropping on growth, quality and land equivalent ratio under semi-arid conditions. Scientia Horticulturae 201:190-198. https://doi.org/10.1016/j.scienta.2016.02.005
Koocheki A, Seyyedi SM, Jamshid Eyni M (2014). Irrigation levels and dense planting affect flower yield and phosphorus concentration of saffron corms under semi-arid region of Mashhad, North. Iran. Scientia Horticulturae 180:147-155. https://doi.org/10.1016/j.scienta.2016.02.005
Kooshki F, Reza Niazkar H, Shiraz S, Asghari Azar V, Moghimian M, Karimi A (2020). Fumaria parviflora improves liver damage and lipid profile changes in STZ induced diabetic rats. Journal of Physiology and Pharmacology 24(3):221e9. https://doi.org/10.32598/ppj.24.3.80
Kumar (2009). Calibration and validation of regression model for non-destructive leaf area estimation of saffron (Crocus sativus L.). Scientia Horticulturae 122(1):142-145. https://doi.org/10.1016/j.scienta.2009.03.019
Kumar V, Bhat ZA, Kumar D, Chashoo IA, Shah MY (2011). Pharmacological profile of Crocus sativus-A comprehensive review. Pharmacologyonline 3:799-811. https://doi.org/10.4103/0973-7847.70919
Lagram K, El Caid MB, El Aaouam S, Lachheb M, El Mousadik A, Serghini MA (2016). In vitro shoot regeneration and development of microcorms of Moroccan saffron (Crocus sativus L.). Atlas Journal of Plant Biology 2016:50-55. https://doi.org/10.5147/ajpb.v0i0.113
Lahmass I, Ouahhoud S, Elyoubi M (2018). Evaluation of antioxidant activities of saffron stigma and spath as by-product of Crocus sativus L. Biology and Medicine 3(4):154-158. https://doi.org/10.15406/mojbm.2018.03.00091
Little EL, Magbana ZV, Parrott WA (2000). A protocol for repetitive somatic embryogenesis from mature peanut epicotyl. Plant Cell Reports 19(4):351-357. https://doi.org/10.1007/s002990050739
Lopresti AL, Drummond PD (2014). Saffron (Crocus sativus) for depression: a systematic review of clinical studies and examination of underlying antidepressant mechanisms of action. Human Psychopharmacology 29(6):517-27. https://doi.org/10.1002/hup.2434
Madubanya LA, Makunga NP, Fennell CW (2006). Dierama luteoalbidum: liquid culture provides an efficient system for the ex-situ conservation of an endangered and horticulturally valuable plant. South African Journal of Botany 72(4):584-588. https://doi.org/10.1016/j.sajb.2006.04.002
Magesh V, Singh JPV, Selvendiran K, Ekambara MG, Sakthisekaran D (2006). Antitumor activity of crocetin in accordance to tumor incidence, antioxidant status, drug metabolizing enzymes and histopathological studies. Molecular and Cellular Biochemistry 287(1-2):127-135. https://doi.org/10.1007/s11010-005-9088-0
Maggi L, Carmona M, Kelly SD, Marigheto N, Alonso GL (2011). Geographical origin, differentiation of saffron spice (Crocus sativus L. stigmas)-Preliminary investigation using chemical and multi-element (H,C,N) stable isotope analysis. Food Chemistry 128(2):543-548. https://doi.org/10.1016/j.foodchem.2011.03.063.
Majourhat K, Martínez-Gómez P, Fernandez JA, Piqueras A (2007). Enhanced plantlet regeneration from cultured meristems in sprouting buds of saffron corms. Acta Horticulturae 739:275-278. https://doi.org/10.17660/ActaHortic.2007.739.34
MalekZadeh S, Khosrowshahli M, Taeb M (2009). Cryopreservation of the axial meristem of Crocus sativus L. Cryobiology 59(3):370-418. https://doi.org/10.1016/j.cryobiol.2009.10.163
Mamoulakis M, Tsarouhas CK (2020). Crocin: a fighter against inflammation and pain. Food and Chemical Toxicology 143:111521. https://doi.org/10.1016/j.fct.2020.111521
Melnyk JP, Wang S, Marcone MF (2010). Chemical and biological properties of the world’s most expensive spice: saffron. Food Research International 43(8):1981-1989. https://doi.org/10.1016/j.foodres.2010.07.033
Mertes PM, Collange O, Coliat P, Banerjee M, Diringer MC, Roche A, … Pivot X (2021). Liposomal encapsulation of trans-crocetin enhances oxygenation in patients with COVID-19-related ARDS receiving mechanical ventilation. Journal of Controlled Release 336:252-261. https://doi.org/10.1016/j.jconrel.2021.06.033
Milajerdi A, Jazayeri S, Hashemzadeh N, Shirzadi E, Derakhshan Z, ADjazayeri B, Akhondzadeh S (2018). The effect of saffron (Crocus sativus L.) Hydroalcoholic extract on metabolic control in type 2 diabetes mellitus: a triple-blinded randomized clinical trial. Journal of Research in Medical Sciences 23:16. https://doi.org/10.4103/jrms.JRMS_286_17
Mir JI, Ahmed N, Shafi W, Rashid R, Khan MH, Sheikh MA, Shah UN, Zaffar S, Rather I (2014). In vitro development and regeneration of microcorms in saffron (Crocus sativus L.). African Journal of Biotechnology 13(26):2637-2640. https://doi.org/10.5897/AJB2013.12831
Mir JI, Ahmed N, Wafai AH, Shafi W, Qadri RA (2018). Relative quantification of apocarotenoids in saffron (Crocus sativus L.). Acta Horticulturae 1200:41-46. https://doi.org/10.17660/ActaHortic.2018.1200.6
Mirhadi E, Nassirli H, Malaekeh-Nikouei B (2020). An updated review on therapeutic effects of nanoparticle-based formulations of saffron components (safranal, crocin, and crocetin). Journal of Pharmaceutical Investigation 50:47-58. https://doi.org/10.1007/s40005-019-00435-1
Mobasseri M, Ostadrahimi A, Tajaddini A, Asghari S, Barati M, Akbarzadeh M, … Alamdari NM (2020). Effects of saffron supplementation on glycemia and inflammation in patients with type 2 diabetes mellitus: A randomized double-blind, placebo-controlled clinical trial study. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 14(2020):527-e534. https://doi.org/10.1016/j.dsx.2020.04.031
Modaghegh MH, Shahabian M, Esmaeili HA (2008). Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytome 15:1032-1037. https://doi.org/10.1016/j.phymed.2008.06.003
Moghadam BH, Bagheri R, Roozbeh B, Ashtary-Larky D, Gaeini AA, Dutheil F, Wong A (2021). Impact of saffron (Crocus Sativus Linn) supplementation and resistance training on markers implicated in depression and happiness levels in untrained young males. Physiology & Behavior 233(2021):113352. https://doi.org/10.1016/j.physbeh.2021.113352
Mohajeri SA, Hedayati N, Bemani-Naeini M (2020). Available saffron formulations and product patents. In: Saffron. Woodhead Publishing, pp 493-515. https://doi.org/10.1016/B978-0-12-818638-1.00034-4
Mokhtari-Zaer A, Saadat S, Ghorani V, Memarzia A, Boskabady MH (2020). The effects of saffron (Crocus sativus) and its constituents on immune system: experimental and clinical evidence. In: Saffron. Academic Press 2020:193-217. https://doi.org/10.1016/B978-0-12-818462-2.00016-4
Musazadeh V, Zarezadeh M, Faghfouri AH, Keramati M, Ghoreishi Z, Farnam A (2022). Saffron, as an adjunct therapy, contributes to relieve depression symptoms: An umbrella meta-analysis. Pharmacological Research 175:105963. https://doi.org/10.1016/j.phrs.2021.105963
Muzaffar S, Rather SA, Khan KZ, Akhter R (2015). Nutritional composition and in-vitro antioxidant properties of two cultivars of Indian saffron. Journal of Food Measurement and Characterization 10(1):185-192. https://doi.org/10.1007/s11694-015-9292-x
Mzabri I, Addi M, Berrichi A (2019). Traditional and modern uses of saffron (Crocus Sativus). Cosmetics 6:63. https://doi.org/10.3390/cosmetics6040063
Mzabria I, Legsayer M, Aliyat F, Maldani M, Kouddane N, Boukroute A, Bekkouch I, Berrichi A (2017). Effect of salt stress on the growth and development of saffron (Crocus sativus L.) in eastern Morocco. Acta Horticulturae 1184:55-62. https://doi.org/10.17660/ActaHortic.2017.1184.8
Nair SC, Kurumboor SK, Hasegawa JH (1995). Saffron chemoprevention in biology and medicine: A review. Cancer Biotherapy 10 (4):257-264. https://doi.org/10.1089/cbr.1995.10.257
Namin MH, Azari N (2017). Effect of nano-silver particles on saffron corm treated with NaCl. Acta Horticulturae 1184:195-210.
Namin MH, Ebrahimzadeh H, Ghareyazie B, Radjabian T, Namin HH (2010). Initiation and origin of stigma-like structures (SLS) on ovary and style explants of saffron in tissue culture. Acta Biologica Cracoviensia / Series Botanica 52(1):55-60. https://doi.org/10.2478/v10182-010-0007-7
Nassar R, Samar Eid S, Chahine R, Chabi B, Bonnieu A, El Sabban M (2020). Antioxidant effects of Lebanese Crocus sativus L. and its main components, crocin and safranal, on human skeletal muscle cells. European Journal of Integrative Medicine 40:101250. https://doi.org/10.1016/j.eujim.2020.101250
Nehvi FA, Khan MA, Lone AA (2010). Effect of radiation and chemical mutagens on variability in saffron (Crocus sativus L.). Acta Horticulturae 850:7-74. https://doi.org/10.17660/ActaHortic.2010.850.8
Noori SMA, Hashemi M, Ghasemi S (2022). A comprehensive review of minerals, trace elements, and heavy metals in saffron. Current Pharmaceutical Biotechnology 23(11):1327-1335. https://doi.org/10.2174/1389201023666220104142531
Parray JA, Kamili AN, Hamid R, Husaini AM (2012). In vitro cormlet production of saffron (Crocus sativus L. Kashmirianus) and their flowering response under greenhouse. GM Crops & Food: Biotechnology in Agriculture and the Food Chain 3(4):289-295. https://doi.org/10.4161/gmcr.21365.
Perera D, Barnes DJ, Baldwin BS, Reichert NA (2015). Mutagenesis of in vitro cultures of Miscanthus 9 giganteus cultivar freedom and detecting polymorphisms of regenerated plants using ISSR markers. Industrial Crops and Products 65:110-116. https://doi.org/10.1016/j.indcrop.2014.12.005
Pierlot G (1925). Le safran. La Chimicae l'Industria 14:1-12.
Premkumar K, Abraham SK, Santhiya ST, Gopinath PM, Ramesh A (2003). Protective effects of saffron (Crocus sativus Linn.) on genotoxins-induced oxidative stress in Swiss albino mice. Drug and Chemical Toxicology 17:614-617. https://doi.org/10.1002/ptr.1209
Premkumar K, Thirunavukkarasu C, Abraham SK, Santhiya ST, Ramesh A (2006). Protective effect of saffron (Crocus sativus L.) aqueous extract against genetic damage induced by anti-tumor agents in mice. Human. Human & Experimental Toxicology 25(2):79-84. https://doi.org/10.1191/0960327106ht589oa
Rajabpoor SH, Azghandi AV, Saboora A (2007). Effects of different concentrations of 2,4-D and BAP on somatic embryogenesis induction in saffron (Crocus sativus L.). Pakistan Journal of Biological Sciences 10(21):3927-3930. https://doi.org/10.3923/pjbs.2007.3927.3930
Rajaei SM, Niknam V, Seyedi SM Ebrahimzadeh H, Razavi K (2009) Contractile roots are the most sensitive organ in Crocus sativus to salt stress. Biologia Plantarum 53(3):523-529. https://doi.org/10.1007/s10535-009-0095-y
Razavia BM, Hossein A, Hosseinzadeh H (2021). Antidepressant activity of Crocus sativus L. and its main constituents: A review. The Neuroscience of Depression Genetics, Cell Biology, Neurology, Behaviour, and Diet 2021:493-502. https://doi.org/10.1016/B978-0-12-817935-2.00045-3
Razem A, El-Kereamy A, Abrams SR, Hill RD (2006). The RNA-binding protein FCA is an abscisic acid receptor. Nature 439(7074):290-294. https://doi.org/10.1038/nature04373
Renau-Morata B, Nebauer SG, Sánchez M, Molina RV (2012). Effect of corm size, water stress and cultivation conditions on photosynthesis and biomass partitioning during the vegetative growth of saffron (Crocus sativus L.). Industrial Crops and Products 39:40-46. https://doi.org/10.1016/j.indcrop.2012.02.009
Renau-Morataa B, Moyáa L, Nebauera SG Seguí-Simarrob JM, Parra-Vegab V, Gómezc MD, Molinaa RV (2013). The use of corms produced under storage at low temperatures as a source of explants for the in vitro propagation of saffron reduces contamination levels and increases multiplication rates. Industrial Crops and Products 46:97-104. https://doi.org/10.1016/j.indcrop.2013.01.013
Rubio-Moraga A, Castillo-Lapez R, Gamez-Gamez L, Ahrazem O (2009). Saffron is a monomorphic species as revealed by RAPD, ISSR and microsatellite analyses. BMC Research Notes 2(1):189. https://doi.org/10.1186/1756-0500-2-189
Sah G, Mokhtari F, Rahimmalek M (2012). Phenolic compounds and antioxidant activity from saffron (Crocus sativus L.) petal. The Journal of Agricultural Science 4(10):175-181. https://doi.org/10.5539/jas.v4n10p175
Salomi MJ, Nair SC, Panikkar KR (1991). Inhibitory effects of Nigella sativa and saffron (Crocus sativus) on chemical carcinogenesis in mice. Nutrition and Cancer 16:67-72. https://doi.org/10.1080/01635589109514142
Salwee Y, Nehvi FA (2014). Creation of genetic variability in saffron (Crocus sativus L.) using induced mutation. Journal of Cell and Tissue Research 14(2):4455-4461.
Samarghandian S, Azimi-Nezhad M, Farkhondeh T (2017). Immunomodulatory and antioxidant effects of saffron aqueous extract (Crocus sativus L.) on streptozotocin-induced diabetes in rats. Indian Heart Journal 69:151-159. https://doi.org/10.1016/j.ihj.2016.09.008
Sarhan AZ, Habib AM, Refaat AM, Awadalla SSA (2013). Comparative effects of BAP and NAA on explant development of micropropagated saffron (Crocus sativus L) corms. Journal of Applied Sciences Research 9(8):5141-5145.
Schmidt M, Betti G, Hensel A (2007). Saffron in phytotherapy: Pharmacology and clinical use. Wiener Medizinische Wochenschrift 157:315-319. https://doi.org/10.1007/s10354-007-0428-4
Sedighara P (2003). Medical characteristics of saffron. Tertian National Symposium on saffron. Mashhad pp. 368-382
Serrano‐Díaz J, Sánchez AM, Maggi L, Martínez‐Tomé M, García‐Diz L, Murcia MA, Alonso GL (2012). Increasing the applications of Crocus sativus flowers as natural antioxidants. Journal of Food Science 77(11):1162-1168. https://doi.org/10.1111/j.1750-3841.2012.02926.x
Sevindik B, Yalcin Mend Y (2016). Somatic Embryogenesis in Crocus sativus L. in vitro embryogenesis in higher plants. Methods in Molecular Biology 1359:351-357. https://doi.org/10.1007/978-1-4939-3061-6_16
Shah W, Hillman T, Playford ED, Hishmeh L (2021). Managing the long-term effects of COVID-19: summary of NICE, SIGN, and RCGP rapid guideline. British Medical Journal 372:n136. https://doi.org/10.1136/bmj.n136
Shahabzadeh Z, Heidari B, Dadkhodaie A (2013). Regenerating salt tolerant saffron (Crocus sativus) using tissue culture with increased pharmaceutical ingredients. Journal of Crop Science and Biotechnology 16(3):209-217. https://doi.org/10.1007/s12892-013-0031-8
Sharifi G, Ebrahimzadeh H (2010). Changes of antioxidant enzyme, activities and isoenzyme profiles during in vitro shoot formation in saffron (Crocus sativus L.). Acta Biologica Hungarica 61(1):73-89. https://doi.org/10.1556/ABiol.61.2010.1.8
Sharifi G, Ebrahimzadeh H, Ghareyazie B, Gharechahi J, Vatankhah E (2012). Identification of differentially accumulated proteins associated with embryogenic and non-embryogenic calli in saffron (Crocus sativus L.). Proteome Science 10(3):1-15. https://doi.org/10.1186/1477-5956-10-3
Sharma KD, Piqueras A (2010). Saffron (Crocus sativus L.) tissue culture: micropropagation and secondary metabolite production. Functional Plant Science and Biotechnology 4(1):15-24.
Sharma KD, Singh BM, Sharma TR, Rathour R, Sharma R, Goel S (2005). Development of low-cost media for in vitro shoot regeneration in saffron (Crocus sativus L.). Indian Perfumer 9:333-337. https://doi.org/10.5147/ajpb.v0i0.113
Sharma KD, Singh BM, Sharma TR, Rathour R, Sharma R, Goel S (2008). In vitro cormlet development in Crocus sativus. Biologia Plantarum 52(4):709-712. https://doi.org/10.4161/gmcr.21365
Sharma P, Bhatt D, Zaidi MG (2012). Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Applied Biochemistry and Biotechnology 167(8):2225-2233. https://doi.org/10.1007/s12010-012-9759-8
Sheibani M, Nemati SH, Davarinejad GH, Azghandi AV, Habashi AA (2007). Induction of somatic embryogenesis in saffron using thidiazuron (TDZ). Acta Horticulturae 739:259-267. https://doi.org/10.3923/pjbs.2007.3564.3570
Simona L, Cerasela P, Florina F, Lazar A, Giancarla V, Danci M, Maria B (2013). In vitro regeneration of Crocus sativus L. The Journal of Horticultural Science and Biotechnology 17:244-247. https://doi.org/10.1016/j.jarmap.2015.07.003
Slackand SA, Tufford LA (1995). Meristem culture for virus elimination.In: Gamborg OL, Phillips GC (Eds). Plant Cell, Tissue and Organ Culture Fundamental Methods, Springer, Berlin. Germany pp 117-128.
Sobolev AP, Carradori S, Capitani D, Vista S, Trella A, Marini F, Mannina L (2014). Saffron samples of different origin: an NMR study of microwave-assisted extracts. Foods 3(3):403-419. https://doi.org/10.3390/foods3030403
Sorooshzadeh A, Hazrati S, Oraki H, Govahi M, Ramazani A (2012). Foliar application of nano-silver influence growth of saffron under flooding stress. Brno Czech Repub EU.
Srivastava R, Ahmed H, Dixit RK, Dharamveer Saraf SA (2010). Crocus sativus L.: A comprehensive review. Pharmacognosy Reviews 4(8):200-208. https://doi.org/10.4103/0973-7847.70919
Staikidou L, Watson S, Harvey BMR, Selby C (2005). Narcissus bulblet formation in vitro: effects of carbohydrate type and osmolarity of the culture medium. Plant Cell, Tissue and Organ Culture 80(3):313-320. https://doi.org/10.1007/s11240-004-1366-0
Tarantilis PA, Polissiou M (2997). Isolation and identification of the aroma components from saffron (Crocus sativus L.). Journal of Agricultural and Food Chemistry 45:459-462.
Termentzi A, Kokkalou E (2008) LC-DAD-MS (ESI+) analysis and antioxidant capacity of Crocus sativus petal extracts. Planta Medica 74(5):573-581. https://doi.org/10.1055/s-2008-1074498
Torbaghan ME, Ahmadi MM (2011). The effect of salt stress on flower yield and growth parameters of saffron (Crocus sativus L.) in greenhouse condition. International Research Journal of Agricultural Science and Soil Science 1(10):421-427.
Tsatsaroni EG, Liakopoulou-Kyriakides M, Eleftheriadis IC (1998). Comparative study of dyeing properties of two yellow natural pigments. Effect of enzymes and proteins. Dyes and Pigments 37(4):307-315.
Vahedi M, Kalantari S, Alireza Salami S (2015). Effects of Osmolytic Agents on Somatic Embryogenesis of Saffron (Crocus sativus L.). Notulae Scientia Biologicae 7:57-61. https://doi.org/10.15835/nsb719442
Vatankhah E, Niknam V, Ebrahimzadeh H (2010). Activity of antioxidant enzyme during in vitro organogenesis in Crocus sativus. Biologia Plantarum 54(3):509-514. https://doi.org/10.1007/s10535-010-0089-9
Vatankhah E, Niknam V, Ebrahimzadeh H (2014). Histological and biochemical parameters of Crocus sativus during in vitro root and shoot organogenesis. Biologia Plantarum 58:201-208. https://doi.org/10.1007/s10535-013-0388-z
Wali AF, Pillai JR, Al Dhaheri Y, Rehman MU, Shoaib A, Sarheed O, ... Ahmad P (2020). Crocus sativus L. extract containing polyphenols modulates oxidative stress and inflammatory response against anti-tuberculosis drugs-induced liver injury. Plants 9:167. https://doi.org/10.3390/plants9020167
Yarami N, Sepaskhah AR (2015). Saffron response to irrigation water salinity, cow manure and planting method. Agricultural Water Management 150:57-66. https://doi.org/10.1016/j.agwat.2014.12.004
Zaffar G, Ahmad M, Shahida I, Razvi SM, Habib M, Ahmad A (2014). Effect of paclobutrazol and sucrose on in vitro corm formation in saffron (Crocus sativus). Journal of Cell & Tissue Research 14 :4069-4072.
Zaffer G, Wani SA, Anjum T, Zeerak NA (2004). Colchicine induced variability in saffron. Acta Horticulturae 650:277-288. https://doi.org/10.17660/ActaHortic.2004.650.31
Zeka K, Ruparelia KC, Continenza MA, Stagos D, Vegliò F, Arroo RR (2015). Petals of Crocus sativus L. as a potential source of the antioxidants crocin and kaempferol. Fitoterapia 107:128-134. https://doi.org/10.1016/j.fitote.2015.05.014
Zeng Y, Yan F, Tang L, Chen F (2003). Increased crocin production and induction frequency of stigma-like- structure from floral organs of Crocus sativus byprecursor feeding. Plant Cell, Tissue and Organ Culture 72(2):185-191. https://doi.org/10.1023/A:1022215021613
Zeybek E, Önde S, Kaya Z (2012). Improved in vitro micropropagation methods with adventitious corms and roots for endangered saffron. Central European Journal of Biology 7(1):138-145. https://doi.org/10.2478/s11535-011-0102-0
Zhang A, Shen Y, Cen M, Hong X, Shao Q, Chen Y, Zheng B (2019). Polysaccharide and crocin contents, and antioxidant activity of saffron from different origins. Industrial Crops and Products 133:111-117. https://doi.org/10.1016/j.indcrop.2019.03.009
Zhao J, Chen F, Yan F, Tang L, Xu Y (2001). In vitro regeneration of style-stigma-like structure from stamens of Crocus sativus. Acta Botanica Sinica 43(5):475-479.
Downloads
Published
How to Cite
Issue
Section
License
Papers published in Notulae Scientia Biologicae are Open-Access, distributed under the terms and conditions of the Creative Commons Attribution License.
© Articles by the authors; licensee SMTCT, Cluj-Napoca, Romania. The journal allows the author(s) to hold the copyright/to retain publishing rights without restriction.
License:
Open Access Journal - the journal offers free, immediate, and unrestricted access to peer-reviewed research and scholarly work, due SMTCT supports to increase the visibility, accessibility and reputation of the researchers, regardless of geography and their budgets. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author.













.png)















