Phytochemical screening, antimicrobial, and antioxidant properties of Helianthus annuus and Hyophila involuta: A comparative account
DOI:
https://doi.org/10.55779/nsb16211557Keywords:
angiosperms, antimicrobial, antioxidant, bryophytes, phytochemicalsAbstract
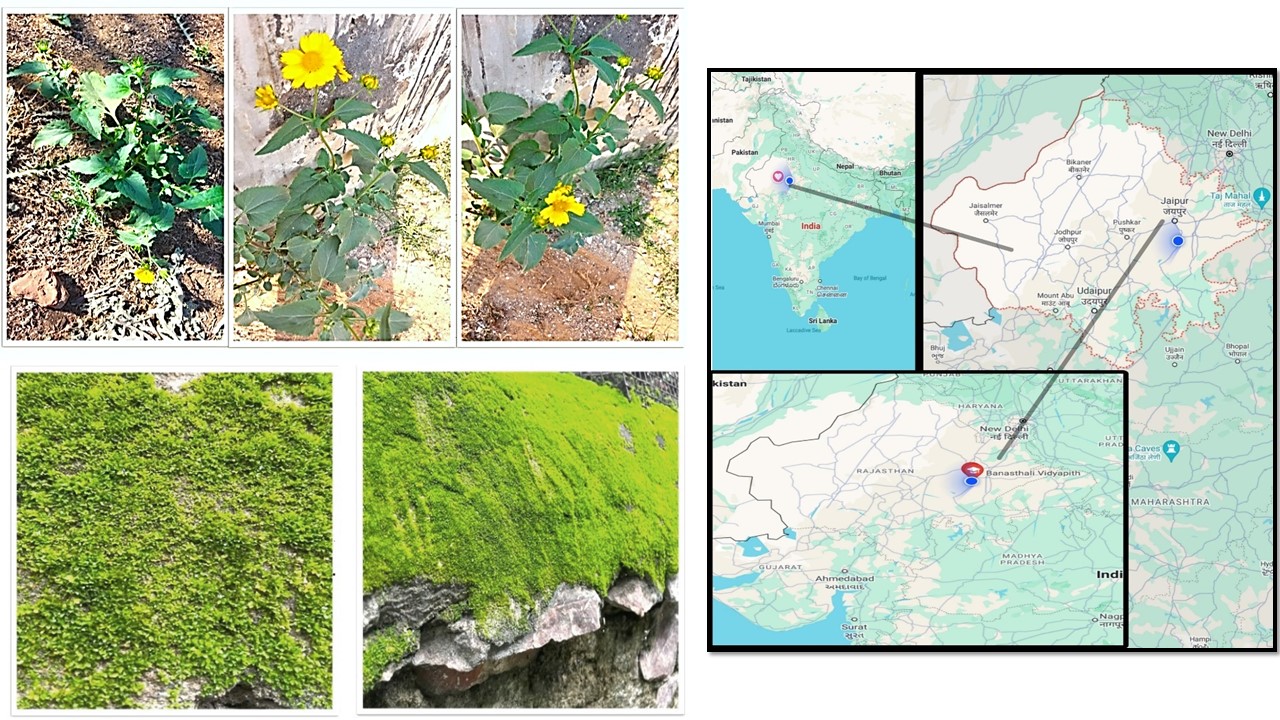
Angiosperms and bryophytes, though distantly related plant groups, have many similar ecological and economic implications, including medicinal value. Therefore, this study aimed to analyse the phenolic and flavonoids composition and antioxidant and antimicrobial activities of Helianthus annuus L. (Angiosperms-Asteraceae) and another plant, a moss (Bryophyta), Hyophila involuta (Hook.) Jaeg., aerial parts (leaves), prepared in four different extracts (methanol, chloroform, distilled water, and petroleum ether). Phytochemical screening was conducted using standard methods of precipitation and colouration reactions. The Folin-Ciocalteu method was employed to determine the total phenol content, while the Aluminium Chloride Colorimetric method was used for flavonoid content determination. The antioxidant activity was measured through two methods: DPPH and NOSA scavenging activity. The phytochemical screening detected the presence and absence of fixed oils and fats, flavonoids, saponins, terpenoids, tannins, polyphenols, carbohydrates, and glycosides in both plants. The antibacterial and antifungal activity of both plants' methanolic extracts was examined against bacterial and fungal pathogens, i.e., Escherichia coli and Bacillus subtilis and fungal strains, i.e., Fusarium oxysporum and Aspergillus niger. The results were compared to a regular antibiotic disc and negative control that served as a methanol solvent. The methanolic extract of H. annuus has higher total phenol and flavonoid content, as well as antioxidant and antimicrobial activity, than H. involuta. Based on these data, it can be concluded that, while H. annuus is more effective than H. involuta, both distantly related plant species have similar phytochemical profiles and should be included equally in future herbal compositions.
Metrics
References
Abubacker MN, Deepalakshmi T (2013). In vitro antifungal potential of bioactive compound methyl ester of hexadecanoic acid isolated from Annona muricatalinn (Annonaceae) leaves. Bioscience Biotechnology Research Asia 10(2):879-884. http://dx.doi.org/10.13005/bbra/1211
Achika JI, Arthur DE, Gerald I, Adedayo A (2014). A review on the phytoconstituents and related medicinal properties of plants in the Asteraceae family. IOSR Journal of Applied Chemistry 7(8):1-8. http://dx.doi.org/10.9790/5736-07810108
Adebiyi AO, Oyedeji AA, Chikwendu EE, Fatoke OA (2012). Phytochemical screening of two tropical moss plants, Thuidium gratum P. Beauv and Barbula indica Brid, grown in the Southwestern ecological zone of Nigeria. American Journal of Analytical Chemistry 3:836-839. http://dx.doi.org/10.4236/ajac.2012.312110
Aiyegoro OA, Okoh AI (2010). Preliminary phytochemical screening and in vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complementary and Alternative Medicine 10(1):21. https://doi.org/10.1186/1472-6882-10-21
Alam A, Shrama V, Rawat KK, Verma PK (2015). Bryophytes – the ignored medicinal plants. SMU Medical Journal 2(1):299-316.
Asakawa Y (2007). Biologically active compounds from bryophytes. Pure and Applied Chemistry 79:557-580. https://doi.org/10.1351/pac200779040557
Azwanida NN (2015). A review on the extraction methods uses in medicinal plants, principle, strength and limitation. Medicinal Aromatic Plants 4(196):2167-0412. http://dx.doi.org/10.4172/2167-0412.1000196
Beike AK, Decker EL, Frank W, Lang D, Vervliet-Scheebaum M, Zimmer AD, Reski R (2010). Applied bryology- bryotehcnology. Tropical Bryology 31:22-32. https://doi.org/10.11646/bde.31.1.7
Chopra RN, Nayar SL, Chopra IC (1956). Glossary of Indian Medicinal Plants. CSIR, New Delhi, pp 256.
D’auria FD, Tecca M, Strippoli V, Salvatore G, Battinelli L, Mazzanti G (2005). Antifungal activity of Lavandula angustifolia essential oil against Candida albicans yeast and mycelial form. Medical Mycology 43(5):391-396. https://doi.org/10.1080/13693780400004810
Ediriweera ERHSS (2007). A review on medicinal uses of weeds in Sri Lanka. Tropical Agricultural Research and Extension 10:11-16. https://doi.org/10.4038/tare.v10i0.1865
Fukumoto LR, Mazza G (2000). Assessing antioxidant and prooxidant activities of phenolic compounds. Journal of Agricultural and Food Chemistry 48(8):3597-3604. https://doi.org/10.1021/jf000220w
Glime JM (1988). Methods in Bryology-Proceedings of the Bryological Methods. Workshop, Mainz. The Hattori Botanical Laboratory, Japan, pp 403.
Jimoh FO, Adedapo AA, Afolayan AJ (2011). Comparison of the nutritive value, antioxidant and antibacterial activities of Sonchus asper and Sonchus oleraceus. Records of Natural Products 5(1):29-42. https://doi.org/10.4314/ajb.v7i18.59254
John LS, Ogle DG, Scianna J, Winslow S, Holzworth LK (2010). Plant materials collection guide. Boise, ID-Salt Lake City, UT-Bozeman, MT 1(8):17.
Joshi AR, Joshi K (2000). Indigenous knowledge and uses of medicinal plants by local communities of the Kali Gandaki Watershed Area, Nepal. Journal of Ethnopharmacology 73(1-2):175-183. https://doi.org/10.1016/S0378-8741(00)00301-9
Mandel JR, Barker MS, Bayer RJ, Dikow RB, Gao TG, Jones KE, … Funk VA (2017). The compositae tree of life in the age of phytogenomic. Journal of Systemic Evolution 55:405-410. https://doi.org/10.1111/jse.12265
Medeiros-Neves B, Teixera HF, Von Poser GL (2018). The genus Pterocaulon (Asteraceae)-a review on traditional medicinal uses, chemical constituents, and biological properties. Journal of Ethnopharmacology 224:451-464. https://doi.org/10.1016/j.jep.2018.06.012
Mehra YK, De K (2017). Determination of phytochemical, total flavonoids and antioxidant activity of methanolic extract of Pisum sativum. International Journal of Innovative Pharmaceutical Sciences and Research 5(8):1-12. https://doi.org/10.21276/IJIPSR.2017.05.08.566
Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamy EN, Bakri MM (2018). Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi Journal of Biological Sciences 25(2):361-366. https://doi.org/10.1016/j.sjbs.2017.02.004
Pal DC, Jain SK (1998). Tribal medicine. Naya Prakash. Calcutta.
Saklani S, Mishra AP, Chandra H, Atanassova MS, Stankovic M, Sati B, … Suleria HAR (2017). Comparative evaluation of polyphenol contents and antioxidant activities between ethanol extracts of Vitex negundo and Vitex trifolia L. leaves by different methods. Plants 6(4):45. https://doi.org/10.3390/plants6040045
Sharma SK, Alam A (2022). Biological prospecting of the “Hidden Diversity” of medicinal plants (Asteraceae) in south-eastern Rajasthan. Geophytology 51(1-2):143-148.
Srinivasan B, Krishnan R, Sundarapandian S (2014). Preliminary phytochemical screening and spectroscopic analysis of Ormocarpum sennoides DC. International Journal of Research in Pharmaceutical Sciences 5:216- 220.
Ugoh SC, Haruna IM (2013). Phytochemical screening and antibacterial activity of the fruit and leaf extracts of Tamarindus indica Linn. Report and Opinion 5(8):18-27.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Supriya K. SHARMA, Afroz ALAM

This work is licensed under a Creative Commons Attribution 4.0 International License.
Papers published in Notulae Scientia Biologicae are Open-Access, distributed under the terms and conditions of the Creative Commons Attribution License.
© Articles by the authors; licensee SMTCT, Cluj-Napoca, Romania. The journal allows the author(s) to hold the copyright/to retain publishing rights without restriction.
License:
Open Access Journal - the journal offers free, immediate, and unrestricted access to peer-reviewed research and scholarly work, due SMTCT supports to increase the visibility, accessibility and reputation of the researchers, regardless of geography and their budgets. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author.












.png)















