Rapid GC-MS based metabolic profiling of Capsicum annuum L. seeds at different phases of germination
DOI:
https://doi.org/10.55779/nsb16211906Keywords:
Capsicum annuum cv. ‘Bullet’, GC-MS, imbibition, seed germination, secondary metabolitesAbstract
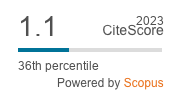
Seed germination is the most critical stage of the life cycle of a plant. Metabolic regulation is significant during the course of seed germination for the establishment of seedlings. Upon imbibition, the dry and fully developed seeds undergo the release of various organic molecules, such as low molecular weight carbonyl compounds in the form of gases and volatiles, as well as water-soluble organic components like enzymes and polysaccharides. Volatile organic compounds may impart both positive and negative influences on seed germination. A metabolite profiling approach based on gas chromatography−mass spectrometry (GC-MS) was used to investigate time-dependent metabolic changes during the germination of Capsicum annuum L. cv. ‘Bullet’. This study aimed to identify bioactive compounds from the methanolic extract of dry, imbibed, germinating seeds and young seedlings of the ‘Bullet’ cultivar of C. annuum L. by GC-MS. A total of 56 distinct categories of compounds were identified in dry seeds, while fully absorbed seeds contained 53 compounds. In the germinating seed, 52 compounds were identified. With regard to immature seedlings, a total of 28 compounds were identified. The analysis revealed that each stage has a unique bio-constituent; only five compounds were detected in all extracts.
Metrics
References
Bardou P, Mariette J, Escudié F, Djemiel C, Klopp C (2014). Jvenn: an interactive Venn diagram viewer. BMC Bioinformatics 15(1):1-7. https://doi.org/10.1186/1471-2105-15-293
Bodoprost J, Rosemeyer H (2007). Analysis of Phenacylester derivatives of fatty acids from human skin surface sebum by reversed-phase HPLC: Chromatographic mobility as a function of physico-chemical properties. International Journal of Molecular Sciences 8(11):1111-1124. https://doi.org/10.3390/i8111111
Chinnasamy G, Sundareswaran S, Subramaniyan K, Raja K, Renganayaki P, Marimuthu S (2022). Volatile organic compound analysis as advanced technology to detect seed quality in groundnut. Journal of Applied and Natural Science 14(3):885-894. https://doi.org/10.31018/jans.v14i3.3617
Doss RP, Kimura Y, Christian JK (1983). Endogenous levels of abscisic acid and decanoic acid in Dutch iris bulbs and the influence of abscisic acid and decanoic acid on iris meristems cultured in vitro. Plant Physiology 72(3):713-716. https://doi.org/10.1104/pp.72.3.713
El-Maarouf-Bouteau H (2022). The seed and the metabolism regulation. Biology (Basel) 11(2):168. https://doi.org/10.3390/biology11020168
Falodun A, Siraj R, Choudhary MI (2009). GC-MS analysis of insecticidal leaf essential oil of Pyrenacantha staudtii Hutch and Dalz (Icacinaceae). Tropical Journal of Pharmaceutical Research 8(2):139-143. https://doi.org/10.4314/tjpr.v8i2.44522
Gomez-Maqueo X, Soriano D, Velázquez-Rosas N Jiménez-Durán K, Garciadiego MdM, Gamboa-de Buen A (2020). The seed water content as a time-independent physiological trait during germination in wild tree species such as Ceiba aesculifolia. Scientific Reports 10:10429 https://doi.org/10.1038/s41598-020-66759-3
Gu E-J, Kim DW, Jang G-J, Song SH, Lee J-I, Lee SB, . . . Kim H-J (2017). Mass-based metabolomic analysis of soybean sprouts during germination. Food Chemistry 217:311-319. https://doi.org/10.1016/j.foodchem.2016.08.113
Gupta E (2020). β-Sitosterol: Predominant phytosterol of therapeutic potential. In: Mishra P, Mishra RR, Adetunji CO (Eds). Innovations in Food Technology: Current Perspectives and Future Goals. Springer, pp 465-477. https://doi.org/10.1007/978-981-15-6121-4_32
Hailstones M, Smith M (1989). Thermally-derived volatile aldehydes in relation to seed viability in soybean seeds. Seed Science and Technology 17(3):649-658.
Han B, Fernandez V, Pritchard HW, Colville L (2021). Gaseous environment modulates volatile emission and viability loss during seed artificial ageing. Planta 253:1-16. https://doi.org/10.1007/s00425-021-03620-5
Huang C-M, Chang C-H, Yang C-S, Lin C-L, Chang T-H, Chen C-L, . . . Chen J-J (2021). A new acenaphthylene and bioactive constituents of Salvia arisanensis. Chemistry of Natural Compounds 57:991-995. https://doi.org/10.1007/s10600-021-03535-3
Hung R, Lee S, Rodriguez-Saona C, Bennett JW (2014). Common gas phase molecules from fungi affect seed germination and plant health in Arabidopsis thaliana. AMB Express 4(1):1-7. https://doi.org/10.1186/s13568-014-0053-8
Inácio MC, Moraes RM, Mendonça PC, Morel LJ, França SC, Bertoni BW, Pereira AM (2013). Phenolic compounds influence seed dormancy of Palicourea rigida H.B.K. (Rubiaceae), a medicinal plant of the Brazilian Savannah. American Journal of Plant Sciences 4(1):129-133. https://doi.org/10.4236/ajps.2013.41017
Karimzadeh H, Farhang H, Rahimmalek M, Esfahani MT (2023). Spatio-temporal variations of extract produced and fatty acid compounds identified of Gundelia tournefortii L. seeds in central Zagros, Iran. Scientific Reports 13(1):7665. https://doi.org/10.1038/s41598-023-34538-5
Kazmi RH, Willems LA, Joosen RV, Khan N, Ligterink W, Hilhorst HW (2017). Metabolomic analysis of tomato seed germination. Metabolomics 13:1-17. https://doi.org/10.1007/s11306-017-1284-x
Khakimov B, Rasmussen MA, Kannangara RM, Jespersen BM, Munck L, Engelsen SB (2017). From metabolome to phenotype: GC-MS metabolomics of developing mutant barley seeds reveals effects of growth, temperature and genotype. Scientific Reports 7(1):8195. https://doi.org/10.1038/s41598-017-08129-0
Krishnaveni M, Dhanalakshmi R, Nandhini N (2014). GC-MS analysis of phytochemicals, fatty acid profile, antimicrobial activity of Gossypium seeds. International Journal of Pharmaceutical Sciences Review and Research 27(1):273-276.
Li C, Cheng X, Jia Q, Song H, Liu X, Wang K, . . . Zhang M (2017). Investigation of plant species with identified seed oil fatty acids in Chinese literature and analysis of five unsurveyed Chinese endemic species. Frontiers in Plant Science 8:224. https://doi.org/10.3389/fpls.2017.00224
Mihaylova D, Popova A, Vrancheva R, Dincheva I (2022). HS-SPME-GC–MS volatile profile characterization of peach (Prunus persica L. Batsch) varieties grown in the Eastern Balkan Peninsula. Plants 11(2):166. https://doi.org/10.3390/plants11020166
Mira S, González-Benito ME, Hill LM, Walters C (2010). Characterization of volatile production during storage of lettuce (Lactuca sativa) seed. Journal of Experimental Botany 61(14):3915-3924. https://doi.org/10.1093/jxb/erq202
Mira S, Hill LM, González-Benito ME, Ibáñez MA, Walters C (2016). Volatile emission in dry seeds as a way to probe chemical reactions during initial asymptomatic deterioration. Journal of Experimental Botany 67(6):1783-1793. https://doi.org/10.1093/jxb/erv568
Motsa M, Slabbert M, Bester C, Mokwena L, Taylor M (2017). Volatile organic compounds from germinating seeds of Cyclopia species as affected by temperature. Seed Science and Technology 45(1):43-55. https://doi.org/10.15258/sst.2017.45.1.22
Nagel M, Börner A (2010). The longevity of crop seeds stored under ambient conditions. Seed Science Research 20(1):1-12. https://doi.org/10.1017/S0960258509990213
Nonogaki H, Bassel GW, Bewley JD (2010). Germination—Still a mystery. Plant Science 179(6):574-581. https://doi.org/10.1016/j.plantsci.2010.02.010
Palekar S, Patel B, Girish N, Menon S (2020). Rapid GC-MS based Phytochemical profiling of extracts of germinating seeds of Dolichos lablab Linn. Journal of Plant Science Research 36(1/2):77-84. http://dx.doi.org/10.32381/JPSR.2020.36.1-2.10
Pan Y, Wang Z, Zhao SW, Wang X, Li YS, Liu JN, . . . Xi JH (2021). The herbivore‐induced plant volatile tetradecane enhances plant resistance to Holotrichia parallela larvae in maize roots. Pest Management Science 78(2):550-560. https://doi.org/10.1002/ps.6660
Probert RJ, Daws MI, Hay FR (2009). Ecological correlates of ex situ seed longevity: a comparative study on 195 species. Annals of Botany 104(1):57-69. https://doi.org/10.1093/aob/mcp082
Radchuk V, Borisjuk L (2014). Physical, metabolic and developmental functions of the seed coat. Frontiers in Plant Science 5:510. https://doi.org/10.3389/fpls.2014.00510
Rahman M, Anwar M (2006). Fungitoxic and cytotoxic activity of a novel compound 1, 2-benzenedicarboxylic acid, diisooctyl ester of Plumbago zeylanica linn. Asian Journal of Microbiology Biotechnology and Environmental Sciences 8(3):461-464.
Rao M, Anilkumar C (2020). Conventional and contemporary approaches to enhance efficiency in breeding chilli/hot pepper. In: Gosal S, Wani S (Eds). Accelerated Plant Breeding. Springer. https://doi.org/10.1007/978-3-030-47298-6_9
Reszczyńska E, Hanaka A (2020). Lipids composition in plant membranes. Cell Biochemistry and Biophysics 78(4):401-414. https://doi.org/10.1007/s12013-020-00947-w
Rohloff J (2015). Analysis of phenolic and cyclic compounds in plants using derivatization techniques in combination with GC-MS-based metabolite profiling. Molecules 20(2):3431-3462. https://doi.org/10.3390/molecules20023431
Saha K, Proma RZ, Khan N (2020). Phytochemical screening of plant extracts and GC-MS analysis of n-hexane extract of the leaves of Cassia alata Linn. The Journal of Phytopharmacology 9(5):342-347. https://doi.org/10.31254/phyto.2020.9509
Sahoo BM, Ravi Kumar BV, Banik BK, Borah P (2020). Polyaromatic hydrocarbons (PAHs): Structures, synthesis and their biological profile. Current Organic Synthesis 17(8):625-640. https://doi.org/10.2174/1570179417666200713182441
Sarkar A, Sadhukhan S (2023). Health benefits of phytochemicals in hot pepper (Capsicum annuum L.). In: Malik JA, Goyal MR, Birwal P, Watharkar RB (Eds). Plant-Based Bioactive Compounds and Food Ingredients. Apple Academic Press, pp 207-236.
Sarkar AK, Sadhukhan S (2022). Proteomics—a powerful tool for understanding saline stress response in germinating seed. In: Roy S, Mathur P, Chakraborty AP, Saha SP (Eds). Plant Stress: Challenges and Management in the New Decade. Springer, pp 375-399. https://doi.org/10.1007/978-3-030-95365-2_24
Sarkar AK, Oraon S, Mondal S, Sadhukhan S (2023). Effect of salinity on seed germination and seedling growth of Bullet cultivar of chilli (Capsicum annuum L.). Brazilian Journal of Botany 1-13. https://doi.org/10.1007/s40415-023-00894-9
Sokmen BB, Hasdemir B, Yusufoglu A, Yanardag R (2014). Some monohydroxy tetradecanoic acid isomers as novel urease and elastase inhibitors and as new antioxidants. Applied Biochemistry and Biotechnology 172:1358-1364. https://doi.org/10.1007/s12010-013-0595-2
Umarani R, Bhaskaran M, Vanitha C, Tilak M (2020). Fingerprinting of volatile organic compounds for quick assessment of vigour status of seeds. Seed Science Research 30(2):112-121. https://doi.org/10.1017/S0960258520000252
Weitbrecht K, Müller K, Leubner-Metzger G (2011). First off the mark: early seed germination. Journal of Experimental Botany 62(10):3289-3309. https://doi.org/10.1093/jxb/err030
Zhang M, Liu Y, Torii I, Sasaki H, Esashi Y (1993). Evolution of volatile compounds by seeds during storage periods. Seed Science and Technology 21(2):359-373.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Anup Kumar SARKAR, Satyajit ORAON, Subir GHOSH, Kakan BALL, Sanjoy SADHUKHAN

This work is licensed under a Creative Commons Attribution 4.0 International License.
Papers published in Notulae Scientia Biologicae are Open-Access, distributed under the terms and conditions of the Creative Commons Attribution License.
© Articles by the authors; licensee SMTCT, Cluj-Napoca, Romania. The journal allows the author(s) to hold the copyright/to retain publishing rights without restriction.
License:
Open Access Journal - the journal offers free, immediate, and unrestricted access to peer-reviewed research and scholarly work, due SMTCT supports to increase the visibility, accessibility and reputation of the researchers, regardless of geography and their budgets. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author.












.png)















