First report of azole-resistant Aspergillus species in poultry feed in Kabale, South West, Uganda
DOI:
https://doi.org/10.55779/nsb16211789Keywords:
azole resistance, environment, fungi, poultryAbstract
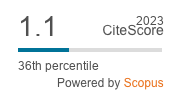
Widespread use of azoles in clinical and environmental settings has favored the selection of azole-resistant Aspergillus species necessitating antifungal susceptibility studies understanding their prevalence and resistance profiles in those settings. However, there is still limited information available on these strains in Africa. The objective of this study was to assess the environmental prevalence of Azole-resistance Aspergillus strains in poultry feed in Kabale, South West Uganda. The study evaluated the susceptibility profile of Aspergillus and other fungi isolates from 10 poultry feeds across 10 different commercial outlets. Isolates were first identified using rDNA 18S genomic sequencing via ITS 1 and 2 primer combination and confirmed using Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF). Seventeen fungal strains were identified of which Aspergillus flavus was the most prevalent. All Aspergillus and one Penicillium isolates were further screened for azole-resistance using azole-containing agar plates (itraconazole, voriconazole, posaconazole, isavuconazole) and fluconazole antifungal susceptibility strip method at 4 mg/L, which was the first line of treatment. Aspergillus chevalieri, A. tamari, A. flavus, P. citrinum fungi isolates showed resistance to fluconazole. The EUCAST susceptibility testing method did not show any of the isolates resistant to tested azoles (itraconazole, voriconazole, posaconazole, isavoriconazole) or amphotericin B. Overall, we found resistance to fluconazole. This study is the first investigative study of Aspergillus species in poultry feed in Kabale, South West, Uganda.
Metrics
References
Alastruey-Izquierdo A, Alcazar-Fuoli L, Cuenca-Estrella M (2014). Antifungal susceptibility profile of cryptic species of Aspergillus. Mycopathologia 178:427-433. https://doi.org/10.1007/s11046-014-9775-z
Amona FM, Oladele RO, Resendiz-Sharpe A, Denning DW, Kosmidis C, Lagrou K, ... Han L (2022). Triazole resistance in Aspergillus fumigatus isolates in Africa: a systematic review. Medical Mycology 60(8):myac059. https://doi.org/10.1093/mmy/myac059.
Ampaire L, Muhindo A, Orikiriza P, Mwanga-Amumpaire J, Bebell L, Boum Y (2016). A review of antimicrobial resistance in East Africa. African Journal of Laboratory Medicine 5:1-6. https://doi.org/10.4102/ajlm.v5i1.432
Arendrup MC (2014). Update on antifungal resistance in Aspergillus and Candida. Clinical Microbiology and Infection 20(6):42-48. https://doi.org/10.1111/1469-0691.12513
Becker P, Normand AC, Vanantwerpen G, Vanrobaeys M, Haesendonck R, Vercammen F, ... Hendrickx M (2019). Identification of fungal isolates by MALDI-TOF mass spectrometry in veterinary practice: validation of a web application. Journal of Veterinary Diagnostic Investigation 31(3)471-474. https://doi.org/10.1177/1040638719835577.
Bhattacharya S, Sae-Tia S, Fries BC (2020). Candidiasis and mechanisms of antifungal resistance. Antibiotics 9(6):312. https://doi.org/10.3390/antibiotics9060312.
Buckley M (2007). The fungal kingdom—diverse and essential roles in earth’s ecosystem. A report based on a colloquium held November 2–4, 2007. Washington, DC: American Academy of Microbiology, 2008.
Bueid A, Howard SJ, Moore CB, Richardson MD, Harrison E, Bowyer P, Denning DW (2010). Azole antifungal resistance in Aspergillus fumigatus: 2008 and 2009. Journal of Antimicrobial Chemotherapy 65(10):2116-2118. http://dx.doi.org/10.1093/jac/dkq279
Byarugaba DK (2004). Antimicrobial resistance in developing countries and responsible risk factors. International Journal of Antimicrobial Agents 24(2):105-110. https://doi.org/10.1016/j.ijantimicag.2004.02.015
Chowdhary A, Sharma C, van den Boom M, Yntema JB, Hagen F, Verweij PE, Meis JF (2014). Multi-azole-resistant Aspergillus fumigatus in the environment in Tanzania. Journal of Antimicrobial Chemotherapy 69(11):2979-2983. https://doi.org/10.1093/jac/dku259
Collignon PJ, McEwen SA (2019). One health—its importance in helping to better control antimicrobial resistance. Tropical Medicine and Infectious Disease 4(1):22. https://doi.org/10.3390/tropicalmed4010022
Cuong NV, Padungtod P, Thwaites G, Carrique-Mas JJ (2018). Antimicrobial usage in animal production: a review of the literature with a focus on low-and middle-income countries. Antibiotics 7(3):75. https://doi.org/10.3390/antibiotics7030075
Dahshan H, Abd-Elall AMM, Megahed AM, Abd-El-Kader MA, Nabawy EE (2015). Veterinary antibiotic resistance, residues, and ecological risks in environmental samples obtained from poultry farms, Egypt. Environmental Monitoring and Assessment 187:1-10. https://doi.org/10.1007/s10661-014-4218-3
Davis RH, Perkins DD (2002). Neurospora: a model of a model microbes. Nature Reviews: Genetics 3:7-13. https://doi.org/10.1038/nrg797
Egbuta MA, Mwanza M, Babalola OO (2017). Health risks associated with exposure to filamentous fungi. International Journal of Environmental Research and Public Health 14(7):719. https://doi.org/10.3390/ijerph14070719
Enany ME, Algammal AM, Nasef SA, Abo-Eillil SA, Bin-Jumah M, Taha AE, Allam AA (2019). The occurrence of the multidrug resistance (MDR) and the prevalence of virulence genes and QACs resistance genes in E. coli isolated from environmental and avian sources. AMB Express 9:1-9. https://doi.org/10.1186%2Fs13568-019-0920-4
FAO (2014). Food and Agriculture Organization. Animal Production and Health Guidelines No. 16; Decision Tools for Family Poultry Development FAO: Rome, Italy, 2014.
Forman S, Plante C, Murray G, Rey B, Belton D, Evans B, Steinmetz P (2012). Position paper: improving governance for effective veterinary services in developing countries--a priority for donor funding. Revue Scientifique et Technique (International Office of Epizootics) 31(2):647-660. https://doi.org/10.20506/rst.31.2.2143
Gerson U, Gafni A, Paz Z, Sztejnberg A (2009). A tale of three acaropathogenic fungi in Israel: Hirsutella, Meira and Acaromyces. Diseases of Mites and Ticks 183-194. https://doi.org/10.1007/s10493-008-9202-6
Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Denning DW (2007). Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology 153:1677-1692. https://doi.org/10.1099/mic.0.2007/007641-0
Homa M, Manikandan P, Szekeres A, Kiss N, Kocsubé S, Kredics L, … Papp T (2019) Characterization of Aspergillus tamarii strains from human keratomycoses: Molecular identification, antifungal susceptibility patterns and cyclopiazonic acid producing abilities. Frontiers in Microbiology 10:2249. https://doi.org/10.3389/fmicb.2019.02249
Ikwap K, Erume J, Owiny DO, Nasinyama GW, Melin L, Bengtsson B, ... Jacobson M (2014). Salmonella species in piglets and weaners from Uganda: prevalence, antimicrobial resistance and herd-level risk factors. Preventive Veterinary Medicine 115(1-2):39-47. https://doi.org/10.1016/j.prevetmed.2014.03.009
Kainz K, Bauer MA, Madeo F, Carmona-Gutierrez D (2020). Fungal infections in humans: the silent crisis. Microbial Cell 7(6):143. https://doi.org/10.15698/mic2020.06.718
Kemoi EK, Nyerere A, Bii CC (2018). Triazole-resistant Aspergillus fumigatus from fungicide-experienced soils in Naivasha subcounty and Nairobi County, Kenya. International Journal of Microbiology 2018. https://doi.org/10.1155/2018/7147938.
Kivumbi MT, Standley CJ (2021). Efforts to identify and combat antimicrobial resistance in Uganda: a systematic review. Tropical Medicine and Infectious Disease 6(2):86. https://doi.org/10.3390/tropicalmed6020086
Laxminarayan R, Van Boeckel T, Teillant A (2015). The economic costs of withdrawing antimicrobial growth promoters from the livestock sector. https://doi.org/10.1787/5js64kst5wvl-en
Martinez M, Lopez-Ribot JL, Kirkpatrick WR, Bachmann SP, Perea S, Ruesga MT, Patterson TF (2002). Heterogeneous mechanisms of azole resistance in Candida albicans clinical isolates from an HIV-infected patient on continuous fluconazole therapy for oropharyngeal candidosis. Journal of Antimicrobial Chemotherapy 49(3):515-524. https://doi.org/10.1093/jac/49.3.515
Mbonye AK, Buregyeya E, Rutebemberwa E, Clarke SE, Lal S, Hansen KS, ... LaRussa P (2016). Prescription for antibiotics at drug shops and strategies to improve quality of care and patient safety: a cross-sectional survey in the private sector in Uganda. BMJ Open 6(3):e010632. https://doi.org/10.1136/bmjopen-2015-010632.
McEwen SA, Collignon PJ (2018). Antimicrobial resistance: a one health perspective. Antimicrobial resistance in bacteria from livestock and companion animals. Microbiology Spectrum 6(2):521-527. https://doi.org/10.1128/microbiolspec.arba-0009-2017
Mortensen KL, Jensen RH, Johansen HK, Skov M, Pressler T, Howard SJ, ... Arendrup MC (2011). Aspergillus species and other molds in respiratory samples from patients with cystic fibrosis: a laboratory-based study with focus on Aspergillus fumigatus azole resistance. Journal of Clinical Microbiology 49(6):2243-2251. http://dx.doi.org/10.1128/JCM.00213-11
Naidu J, Singh SM (1994). Aspergillus chevalieri (Mangin) Thom and Church: a new opportunistic pathogen of human cutaneous aspergillosis: Aspergillus chevalieri (Mangin) Thorn und Church: Ein neuer opportunistischer Erreger von kutaner Aspergillose beim Menschen. Mycoses 37(7‐8):271-274. https://doi.org/10.1111/j.1439-0507.1994.tb00425.x
Negatu B, Kromhout H, Mekonnen Y, Vermeulen R (2016). Use of chemical pesticides in Ethiopia: a cross-sectional comparative study on knowledge, attitude and practice of farmers and farm workers in three farming systems. Annals of Occupational Hygiene 60(5):551-566. https://doi.org/10.1093/annhyg/mew004.
Normand AC, Becker P, Gabriel F, Cassagne C, Accoceberry I, Gari-Toussaint M, Piarroux R (2017). Validation of a new web application for identification of fungi by use of matrix-assisted laser desorption ionization–time of flight mass spectrometry. Journal of Clinical Microbiology 55(9):2661-2670. https://doi.org/10.1128/jcm.00263-17
Odoi R, Joakim M (2019). Anti-microbial resistance in Uganda. Retrieved 2021 May 24 from: https://africa-health.com/wp-content/uploads/2019/02/AH-JAN2019-28-30-AMR.pdf
OECD/FAO (2020). Meat. In: OECD-FAO agricultural outlook 2020-2029. OECD Publishing
Osherov N, Kontoyiannis DP (2016). The anti-Aspergillus drug pipeline: is the glass half full or empty?. Sabouraudia 55(1):118-124. https://doi.org/10.1093/mmy/myw060.
Pennerman KK, Yin G, Glenn AE, Bennett JW (2020). Identifying candidate Aspergillus pathogenicity factors by annotation frequency. BMC Microbiology 20:1-11. https://doi.org/10.1186/s12866-020-02031-y
Perea S, López-Ribot JL, Kirkpatrick WR, McAtee RK, Santillán RA, Martı́nez M, ... Patterson TF (2001). Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrobial Agents and Chemotherapy 45(10):2676-2684. https://doi.org/10.1128/AAC.45.10.2676-2684.2001.
Resendiz Sharpe A, Lagrou K, Meis JF, Chowdhary A, Lockhart SR, Verweij PE, ISHAM/ECMM Aspergillus Resistance Surveillance Working Group (2018). Triazole resistance surveillance in Aspergillus fumigatus. Medical Mycology 56(1):S83-S92. https://doi.org/10.1093/mmy/myx144
Resendiz-Sharpe A, Dewaele K, Merckx R, Bustamante B, Vega-Gomez MC, Rolon M, … Lagrou K (2021). Triazole-Resistance in Environmental Aspergillus fumigatus in Latin American and African countries. Journal of Fungi 7(4):292. https://doi.org/10.3390/jof7040292
Resendiz-Sharpe A, Mercier T, Lestrade PP, van der Beek MT, von dem Borne PA, Cornelissen JJ, ... Lagrou K (2019). Prevalence of voriconazole-resistant invasive aspergillosis and its impact on mortality in haematology patients. Journal of Antimicrobial Chemotherapy 74(9):2759-2766. https://doi.org/10.1093/jac/dkz258
Ritchie H (2017) Meat and dairy production. Our World Data. https://ourworldindata.org/meat-production
Sanglard D, Coste AT, Ferrari S (2009). Antifungal drug resistance mechanisms in fungal pathogens from the perspective of transcriptional gene regulation. FEMS Yeast Research 9:1029-1050. https://doi.org/10.1111/j.1567-1364.2009.00578.x
Singhal N, Kumar M, Kanaujia PK, Virdi JS (2015). MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Frontiers in Microbiology 6:144398. https://doi.org/10.3389/fmicb.2015.00791
Snelders E, Camps SM, Karawajczyk A, Schaftenaar G, Kema GH, Van der Lee HA, ... Verweij PE (2012). Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PloS One 7(3):e31801. https://doi.org/10.1371/journal.pone.0031801
Snelders E, van der Lee HAL, Kuijpers J, Rijs AJM, Varga J, Samson RA, ... Verweij PE (2008). Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Medicine 5(11):e219. http://dx.doi.org/10.1371/journal.pmed.0050219.
Tadesse BT, Ashley EA, Ongarello S, Havumaki J, Wijegoonewardena M, González IJ, Dittrich S (2017). Antimicrobial resistance in Africa: a systematic review. BMC Infectious Diseases 17:1-17. https://doi.org/10.1186/s12879-017-2713-1
The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs for Antifungal Agents, Version 10.0. (2020). Available online: http://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals/
The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs for Antifungal Agents, Version 10.0; EUCAST: Växjö. (2020). Sweden.
Uganda National Academy of Sciences UNAS (2015). Antibiotic Resistance in Uganda: Situation Anaysis; Uganda National Academy of Sciences: Kampala, Uganda. SBN 9789970424108.
Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, ... Cornely OA (2018). Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clinical Microbiology and Infection 24:e1-e38. https://doi.org/10.1016/j.cmi.2018.01.002
Van Boeckel TP, Pires J, Silvester R, Zhao C, Song J, Criscuolo NG, ... Laxminarayan R (2019). Global trends in antimicrobial resistance in animals in low-and middle-income countries. Science 365(6459):eaaw1944. https://doi.org/10.1126/science.aaw1944.
van der Linden JW, Snelders E, Kampinga GA, Rijnders BJ, Mattsson E, Debets-Ossenkopp YJ, ... Verweij PE (2011). Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007–2009. Emerging Infectious Diseases 17(10):1846. https://doi.org/10.3201/eid1710.110226.
van Ingen J, van der Lee HA, Rijs TA, Zoll J, Leenstra T, Melchers WJ, Verweij PE (2015). Azole, polyene and echinocandin MIC distributions for wild-type, TR34/L98H and TR46/Y121F/T289A Aspergillus fumigatus isolates in the Netherlands. Journal of Antimicrobial Chemotherapy 70(1):178-181. http://dx.doi.org/10.1093/jac/dku364 |
Whaley SG, Berkow EL, Rybak JM, Nishimoto AT, Barker KS, Rogers PD (2017). Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Frontiers in Microbiology 7:2173. https://doi.org/10.3389/fmicb.2016.02173
WHO | Antimicrobial Resistance. Retrieved 2021 March 9 from: https://www.who.int/antimicrobial-resistance/en/
World Health Organisation (2022). fungal priority pathogens list to guide research, development and public health action. Geneva: World Health Organization; Licence: CC BY-NC-SA 3.0 IGO
Yerbanga IW, Resendiz-Sharpe A, Bamba S, Lagrou K, Nakanabo Diallo S, Rodriguez-Villalobos H, ... Montesinos I (2021). First investigative study of azole-resistant Aspergillus fumigatus in the environment in Burkina Faso. International Journal of Environmental Research and Public Health 18(5):2250. https://doi.org/10.3390/ijerph18052250
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Adeyinka ODEBODE, Giel VANREPPELEN, Agustin R. SHARPE, Patrick Van DIJCK

This work is licensed under a Creative Commons Attribution 4.0 International License.
Papers published in Notulae Scientia Biologicae are Open-Access, distributed under the terms and conditions of the Creative Commons Attribution License.
© Articles by the authors; licensee SMTCT, Cluj-Napoca, Romania. The journal allows the author(s) to hold the copyright/to retain publishing rights without restriction.
License:
Open Access Journal - the journal offers free, immediate, and unrestricted access to peer-reviewed research and scholarly work, due SMTCT supports to increase the visibility, accessibility and reputation of the researchers, regardless of geography and their budgets. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author.












.png)















