Insecticidal potential of Streptomyces sp. dichloromethane extracts against the cactus cochineal Dactylopius opuntiae (Cockerell)
DOI:
https://doi.org/10.55779/nsb15411574Keywords:
Dactylopius opuntiae, dichloromethane extract, EP bacteria, IPM, Streptomyces sp.Abstract
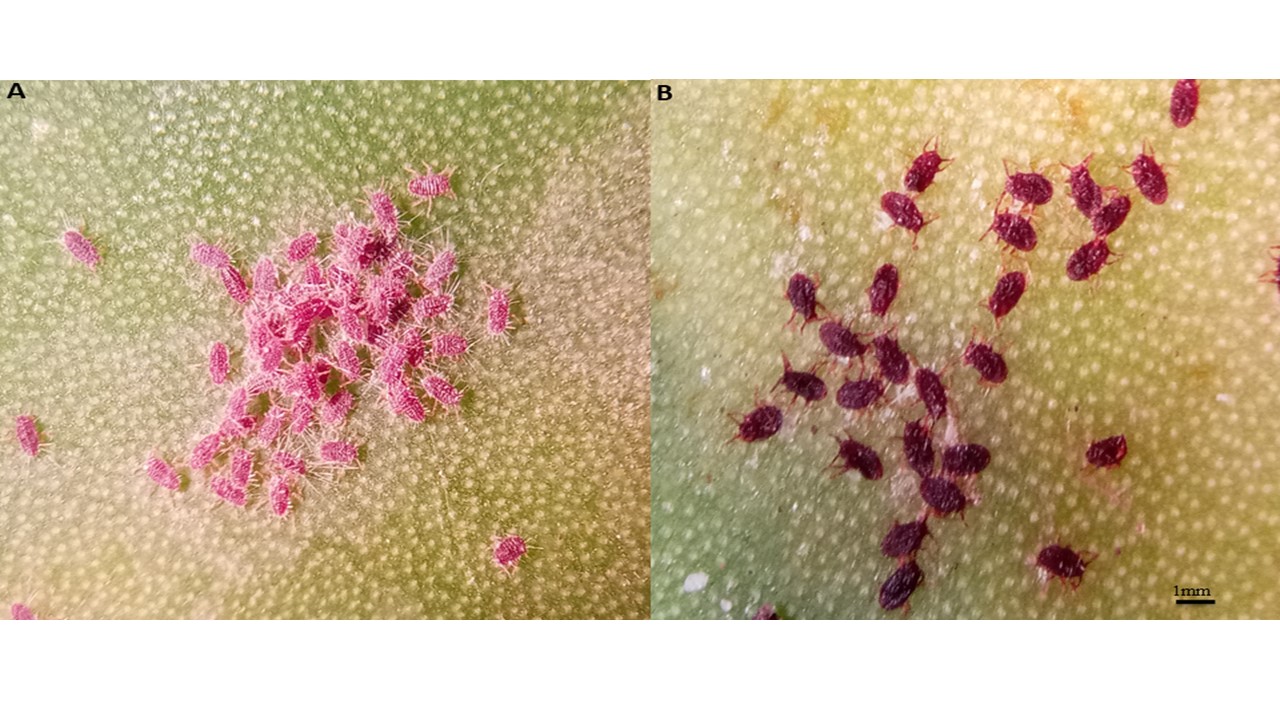
Destructive damages caused by Dactylopius opuntiae (Cockerell) to cactus worldwide require an ecofriendly IPM approach. Streptomyces sp. produce wide range of biologically active secondary metabolites that can be an interesting alternative to chemical insecticides for pest control, as they are less toxic and easily biodegradable. The efficacy of dichloromethane extracts of four Moroccan Streptomyces sp. strains: Streptomyces bellus- E23-2, Streptomyces galilaeus- E23-9, Streptomyces africanus- E23-3, and Streptomyces bellus- E25-12 (applied at 11, 13, 15, 17 and 20 mg mL-1) against D. opuntiae nymphs and adult females was evaluated under laboratory and greenhouse conditions. Results showed that Streptomyces bellus- E23-2 and Streptomyces galilaeus- E23-9 dichloromethane extracts applied at 20 mg mL-1 were more effective, causing higher mortality against nymphs (92% and 91%, respectively) and adult females (90% and 95%, respectively) after 8 days of exposure, resulting in an LT50 value of 3.0 days (nymph), and 3.0 and 6.0 days (adult female), respectively. Streptomyces bellus- E25-12 extract had the lowest mortalities [88% (nymph) and 68% (adult female)]. In greenhouse experiment, the highest first instar nymph mortality was achieved by Streptomyces bellus- E23-2 (55.5%) and Streptomyces galilaeus- E23-9 (50.5%) dichloromethane extracts at 20 mg mL-1. The metabolites found in dichloromethane extracts of Streptomyces bellus- E23-2 and Streptomyces galilaeus- E23-9 show considerable potential to be used in the development of new biopesticide formulations for use in integrated pest management programs against D. opuntiae.
Metrics
References
Abbott WS (1987). A method of computing the effectiveness of an insecticide. 1925. Journal of the American Mosquito Control Association 3(2):302-303.
Aggarwal N, Thind SK, Sharma S (2016). Role of secondary metabolites of actinomycetes in crop protection. In: Gopalakrishnan S, Sathya A, Vijayabharathi R (Eds). Plant Growth-Promoting Actinobacteria. Springer Singapore pp 99-122. https://doi.org/10.1007/978-981-10-0707-1_7
Al-Kaabi FK (2005). Insect Control Using Chitinolytic Soil Actinomycetes as Biocontrol Agents. Theses 594. https://scholarworks.uaeu.ac.ae/all_theses/594
Anderson AJ, Kim YC (2018). Biopesticides produced by plant-probiotic Pseudomonas chlororaphis isolates. Crop Protection 105:62-69. https://doi.org/10.1016/j.cropro.2017.11.009
Aouiche A, Bijani C, Zitouni A, Mathieu F, Sabaou N (2014). Antimicrobial activity of saquayamycins produced by Streptomyces spp. PAL114 isolated from a Saharan soil. Journal de Mycologie Médicale 24(2):17-23. https://doi.org/10.1016/j.mycmed.2013.09.001
Arasu MV, Al-Dhabi NA, Saritha V, Duraipandiyan V, Muthukumar C, Kim SJ (2012). Antifeedant, larvicidal and growth inhibitory bioactivities of novel polyketide metabolite isolated from Streptomyces sp. AP-123 against Helicoverpa armigera and Spodoptera litura. BMC Microbiology 13:1-6. https://doi.org/10.1186/1471-2180-13-105
Becher PG, Keller S, Jung G, Süssmuth RD, Jüttner F (2007). Insecticidal activity of 12-epi-hapalindole J isonitrile. Phytochemistry 68:2493-2497. https://doi.org/10.1016/j.phytochem.2007.06.024
Bérdy J (2005). Bioactive microbial metabolites. The Journal of Antibiotics (Tokyo) 58:1-26. https://doi.org/10.1038/ja.2005.1
Bérdy J (2012). Thoughts and facts about antibiotics: Where we are now and where we are heading. The Journal of Antibiotics (Tokyo) 65:385-395. https://doi.org/10.1038/ja.2012.27
Binod P, Sukumaran RK, Shirke S, Rajput JC, Pandey A (2007). Evaluation of fungal culture filtrate containing chitinase as a biocontrol agent against Helicoverpa armigera. Journal of Applied Microbiology 103:1845-1852. https://doi.org/10.1111/j.1365-2672.2007.03428.x
Bream AS, Ghazal SAEl, El-Aziz ZKA, Ibrahim SY (2001). Insecticidal activity of selected actinomycete strains against the Egyptian cotton leaf worm Spodoptera littoralis (Lepidoptera: Noctuidae). Mededelingen Faculteit Landbouwkundige en Toegepaste Biologische Wetenschappen Universiteit Gent 66:503-512.
Chen CD, Hassandarvish P, Tan GYA, AbuBakar S, Azman AS (2023). Insecticidal activities of Streptomyces sp. KSF103 ethyl acetate extract against medically important mosquitoes and non-target organisms. Scientific Reports 13:4. https://doi.org/10.1038/s41598-022-25387-9
Cokola CM (2019). Monitoring, caractérisation moléculaire et lutte biologique contre Spodoptera frugiperda (Lepidoptera: Noctuidae) [Monitoring, molecular characterization and biological control of Spodoptera frugiperda (Lepidoptera: Noctuidae)]. Université de Liège, Liège, Belgique, pp 79. Travail de fin d’études, Gembloux Agro-Bio Tech. Retrieved 2020 January 10 from: http://matheo.uliege.be/handle/2268.2/8077
Córdova-Kreylos AL, Fernandez LE, Koivunen ME, Yang A, Flor-Weiler LB, Marrone PG (2013). Isolation and characterization of Burkholderia rinojensis sp. nov., a non-Burkholderia cepacia complex soil bacterium with insecticidal and miticidal activities. Applied and Environmental Microbiology 79:7669-7678. https://doi.org/10.1128/AEM.02365-13
Cruz-Rodrílguez JA, González-Machorro E, González A, Ramírez MLR, Lara FM (2016). Autonomous biological control of Dactylopius opuntiae (Hemiptera: Dactyliiopidae) in a prickly pear plantation with ecological management. Environmental Entomology 45:642-648. https://doi.org/10.1093/ee/nvw023
Dhanasekaran D, Sakthi V, Thajuddin N, Panneerselvam A (2010). Preliminary evaluation of Anopheles mosquito larvicidal efficacy of Mangrove actinobacteria. International Journal of Applied Biology and Pharmaceutical Technology 1(2):374-381.
Dhanasekaran D, Thangaraj R (2014). Microbial secondary metabolites are an alternative approach against insect vector to prevent zoonotic diseases. Asian Pacific Journal of Tropical Disease 4(4):253-261. https://doi.org/10.1016/S2222-1808(14)60569-7
El Aalaoui M, Bouharroud R, Sbaghi M, Bouhssini M El, Hilali L, Dari K (2019). Comparative toxicity of different chemical and biological insecticides against the scale insect Dactylopius opuntiae and their side effects on the predator Cryptolaemus montrouzieri. Archives of Phytopathology and Plant Protection 52:155-169. https://doi.org/10.1080/03235408.2019.1589909
Finney DJ (1971). A statistical treatment of the sigmoid response curve. In: Finney DJ (Ed). Probit Analysis. 3rd edn. Cambridge University Press, London pp 333.
Fonseca DF, Salvador ÂC, Santos SA, Vilela C, Freire CS, Silvestre AJ, Rocha SM (2015). Bioactive phytochemicals from wild Arbutus unedo L. berries from different locations in Portugal: Quantification of lipophilic components. International Journal of Molecular Sciences 16(6):14194-14209. https://doi.org/10.3390/ijms160614194
Ganesan P, Stalin A, Paulraj MG, Balakrishna K, Ignacimuthu S, Al-Dhabi NA (2018). Biocontrol and non-target effect of fractions and compound isolated from Streptomyces rimosus on the immature stages of filarial vector Culex quinquefasciatus Say (Diptera: Culicidae) and the compound interaction with Acetylcholinesterase (AChE1). Ecotoxicology and Environmental Safety 161:120-128. https://doi.org/10.1016/j.ecoenv.2018.05.061
Gopalakrishnan S, Rao GVR, Humayun P, Rao VR, Alekhya G, Jacob S, Deepthi K, Vidya MS, Srinivas V, Mamatha L, Rupela OP (2011). Efficacy of botanical extracts and entomopathogens on control of Helicoverpa armigera and Spodoptera litura. African Journal of Biotechnology 10:16667-16673. https://doi.org/10.5897/AJB11.2475
Gopalakrishnan S, Srinivas V, Prasanna SL (2020). Streptomyces. Chapter 5. In: Amaresan N, Kumar MS, Annapurna K, Kumar K, Sankaranarayanan A (Eds). Beneficial Microbes in Agro-Ecology. Academic Press, Amsterdam pp 55-71. https://doi.org/10.1016/B978-0-12-823414-3.00005-8
Gopalakrishnan S, Vadlamudi S, Samineni S, Kumar CVS (2016). Plant growth-promotion and biofortification of chickpea and pigeonpea through inoculation of biocontrol potential bacteria, isolated from organic soils. Springer plus 5:1-11. https://doi.org/10.1186/s40064-016-3590-6
Grzywacz D, Moore D, Rabindra RJ (2014). Mass production of entomopathogens in less industrialized countries. In: Morales-Ramos J, Rojas MG, Shapiro-Ilan D (Eds). Mass Production of Beneficial Organisms: Invertebrates and Entomopathogens. New York: Academic Press, pp 519-561. https://doi.org/10.1016/B978-0-12-391453-8.00015-7
Hemalatha D, Prabhu S, Rani WB, Anandham R (2018). Isolation and characterization of toxins from Xenorhabdus nematophilus against Ferrisia virgata (Ckll.) on tuberose, Polianthes tuberosa. Toxicon 146:42-49. https://doi.org/10.1016/j.toxicon.2018.03.012
Hussain AA, Mostafa SA, Ghazal SA El, Ibrahim SY (2002). Studies on antifungal antibiotic and bioinsecticidal activities of some actinomycete isolates. African Journal of Mycology and Biotechnology 10:63-80.
Jinfeng, EC, Mohamad Rafi MI, Chai Hoon K, Kok Lian H, Yoke Kqueen C (2017). Analysis of chemical constituents, antimicrobial and anticancer activities of dichloromethane extracts of Sordariomycetes sp. endophytic fungi isolated from Strobilanthes crispus. World Journal of Microbiology and Biotechnology 33:1-19. https://doi.org/10.1007/s11274-016-2175-4
Kaur T, Vasudev A, Sohal SK, Manhas RK (2014). Insecticidal and growth inhibitory potential of Streptomyces hydrogenans DH16 on major pest of India, Spodoptera litura (Fab.)(Lepidoptera: Noctuidae). BMC Microbiology 14:1-9. https://doi.org/10.1186/s12866-014-0227-1
Kim JY, Choi JY, Park DH, Park MG, Wang M, Kim HJ, Kim SH, Lee HY, Je YH (2022). Juvenile hormone antagonistic activity of secondary metabolites from Streptomyces lactacystinicus and their insecticidal activity against Plutella xylostella. Journal of Asia-Pacific Entomology 25(2):101870. https://doi.org/10.1016/j.aspen.2022.101870
Krishnamoorthy R, Jose PA, Janahiraman V, Gandhi PI, Gracy RG, Jalali SK, Kumar MS, Malathi VM, Anandham R (2020). Function and insecticidal activity of bacteria associated with papaya mealybug, Paracoccus marginatus Williams & Granara de Willink (Hemiptera: Pseudococcidae). Biocontrol Science and Technology 30:762-778. https://doi.org/10.1080/09583157.2020.1765983
Lacey LA, Grzywacz D, Shapiro-Ilan DI, Frutos R, Brownbridge M, Goettel MS (2015). Insect pathogens as biological control agents: Back to the future. Journal of Invertebrate Pathology 132:1-41. https://doi.org/10.1016/j.jip.2015.07.009
Li X, Qiu Z, Jin Q, Chen G, Guo M (2018). Cell cycle arrest and apoptosis in HT-29 cells induced by dichloromethane fraction from Toddalia asiatica (L.) Lam. Frontiers in Pharmacology 9:629. https://doi.org/10.3389/fphar.2018.00629
Lopes EB, Albuquerque IC, Brito CHDe, Batista JdeL (2009). Velocidade de dispersão de Dactylopius opuntiae em palma gigante (Opuntia fícus- indica). Revista Engenharia Ambiental 6:644-649.
Lopes EB, Emepa Pb (2010). Seleção de Genótipos de Palma Forrageira (Opuntia spp.) e (Nopalea spp.) Resistentes à Cochonilha-do-Carmim (Dactylopius opuntiae Cockerell, 1929) na Paraíba, Brasil [Selection of Forage Palm (Opuntia spp.) and (Nopalea spp.) Genotypes Resistant to the Carmine Cochineal (Dactylopius opuntiae Cockerell, 1929) in Paraíba, Brazil]. Engenharia Ambiental 7:204-215.
Mazzeo G, Nucifora S, Russo A, Suma P (2019). Dactylopius opuntiae, a new prickly pear cactus pest in the Mediterranean: an overview. Entomologia Experimentalis et Applicata 167:59-72. https://doi.org/10.1111/eea.12756
Okongo RN, Puri AK, Wang Z, Singh S, Permaul K (2019). Comparative biocontrol ability of chitinases from bacteria and recombinant chitinases from the thermophilic fungus Thermomyces lanuginosus. Journal of Bioscience and Bioengineering 127:663-671. https://doi.org/10.1016/j.jbiosc.2018.11.007
Osman, Mohamed SH (2008). Antagonistic and insecticidal activities of some streptomyces isolates. Pakistan Journal of Biotechnology 4(1-2):65-71.
Pantoja-Pulido KD, Rodríguez J, Isaza-Martínez JH, Gutiérrez-Cabrera M, Colmenares-Dulcey AJ, Montoya-Lerma J (2020). Insecticidal and cholinesterase activity of dichloromethane extracts of Tithonia diversifolia on Atta cephalotes worker ants (Formicidae: Myrmicinae). Insects 11(3):180. https://doi.org/10.3390/insects11030180
Péchy-Tarr M, Bruck DJ, Maurhofer M, Fischer E, Vogne C, Henkels MD, Donahue KM, Grunder JM, Loper JE, Keel C (2008). Molecular analysis of a novel gene cluster encoding an insect toxin in plant-associated strains of Pseudomonas fluorescens. Environmental Microbiology 10:2368-2386. https://doi.org/10.1111/j.1462-2920.2008.01662.x
Perez-Ramírez, Castrejón-Ayala F, Jiménez-Pérez A (2014). Potential of terpenoids and mealybug extract to deter the establishment of Dactylopius opuntiae (Hemiptera: Dactylopiidae) crawlers on Opuntia ficus-indica. Florida Entomologist 97:269-271.
Portillo MI, Vigueras AL (2006). A review on the cochineal species in Mexico, hosts and natural enemies. Acta Horticulturae 728:249-256. http://dx.doi.org/10.17660/ActaHortic.2006.728.35
Rahoo AM, Mukhtar T, Gowen S, Rahoo RK, Abro SI (2017). Reproductive potential and host searching ability of entomopathogenic nematode, Steinernema feltiae. Pakistan Journal of Zoology 49:229-234. http://dx.doi.org/10.17582/journal.pjz/2017.49.1.229.234
Ramdani C, Bouharroud R, Sbaghi M, Mesfioui A, Bouhssini MEl (2020). Field and laboratory evaluations of different botanical insecticides for the control of Dactylopius opuntiae (Cockerell) on cactus pear in Morocco. Journal of Tropical Insect Science 41:1623-1632. https://doi.org/10.1007/s42690-020-00363-w
Rammali S, Hilali L, Dari k, Bencharki B, Rahim A, Timinouni M, Gaboune F, El Aalaoui M, khattabi A (2022). Antimicrobial and antioxidant activities of Streptomyces species from soils of three different cold sites in the Fez-Meknes region Morocco. Scientific Reports 12:17233. https://doi.org/10.1038/s41598-022-21644-z
Rishikesh GDR, Haque MA, Islam MAU, Rahman MM, Banu MR (2013). In-vitro insecticidal activity of crude extracts of Streptomyces sp. against larvae of Sitophilus oryzae. Journal of Drug Discovery Therapeutics 1(8):60-63.
Rocha JDS (2012). Palma Forrageira no Nordeste do Brasil: Estado da Arte [Fodder Palm in Northeast Brazil: State of the Art]. Embrapa Caprinos e Ovinos pp 40.
Sabbahi R, Hock V (2022). Control of the prickly pear cochineal, Dactylopius opuntiae (Cockerell), in Morocco: an overview. Journal of Plant Diseases and Protection 129(6):1323-1330. https://doi.org/10.1007/s41348-022-00655-y
Sahayaraj K, Subash N, Allingham RW, Kumar V, Avery PB, Mehra LK, McKenzie CL, Osborne LS (2018). Lethal and sublethal effects of three microbial biocontrol agents on Spodoptera litura and its natural predator Rhynocoris kumarii. Insects 9:101. https://doi.org/10.3390/insects9030101
Sbaghi M, Bouharroud R, Boujghagh M, El Bouhssini M (2019). Sources de résistance d’Opuntia spp. contre la cochenille à carmin, Dactylopius opuntiae, au Maroc [Sources of resistance of Opuntia spp. against the carmine scale, Dactylopius opuntiae, in Morocco]. EPPO Bulletin 49(3):585-592. https://doi.org/10.1111/epp.12606
Schneider M, Smagghe G, Viñuela E (2004). Comparative effects of several insect growth regulators and spinosad on the different developmental stages of the endoparasitoid Hyposoter didymator (Thunberg). IOBC/wprs Bulletin 27(6):13-19.
Singh R, Kapoor V, Kumar V (2011). Production of thermostable, Ca+2-independent, maltose producing $α$-amylase by Streptomyces sp. MSC702 (MTCC 10772) in submerged fermentation using agro-residues as sole carbon source. Annals of Microbiology 62:1003-1012. https://doi.org/10.1007/s13213-011-0340-4
Soliman MAW, Hamza AF, Zahran NF, Bassioni G (2021). Microbiological study and insecticidal potential of purified extract from Streptomyces sp. on the larvae of Galleria mellonella. Journal of Plant Diseases and Protection 128:1565-1574. https://doi.org/10.1007/s41348-021-00508-0
Subramani R, Aalbersberg WGL (2012). Marine actinomycetes: an ongoing source of novel bioactive metabolites. Microbiological Research 167:571-580. https://doi.org/10.1016/j.micres.2012.06.005
Tanada Y, Kaya HK (1993). Insect pathology. San Diego, CA: Academic Press pp 666.
Torres JB, Giorgi JA (2018). Management of the false carmine cochineal Dactylopius opuntiae (Cockerell): perspective from Pernambuco state, Brazil. Phytoparasitica 46:331-340. https://doi.org/10.1007/s12600-018-0664-8
Vigueras AL, Cibrian-tovar J, Pelayo-Ortiz C (2009). Use of botanicals extracts to control wild cochineal (Dactylopius opuntiae Cockerell) on cactus pear. Acta Horticulturae 811:229-234. https://doi.org/10.17660/ActaHortic.2009.811.28
Wang Q, Bosch BJ, Vlak JM, Van Oers MM, Rottier PJM, Van Lent JWM (2016). Budded baculovirus particle structure revisited. Journal of Invertebrate Pathology 134:15-22. https://doi.org/10.1016/j.jip.2015.12.001
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Said RAMMALI, Mohamed EL AALAOUI, Mohamed SBAGHI, Khadija DARI, Bouchaib BENCHARKI, Abdelhamid AZEROUAL, Abdelkrim KHATTABI

This work is licensed under a Creative Commons Attribution 4.0 International License.
Papers published in Notulae Scientia Biologicae are Open-Access, distributed under the terms and conditions of the Creative Commons Attribution License.
© Articles by the authors; licensee SMTCT, Cluj-Napoca, Romania. The journal allows the author(s) to hold the copyright/to retain publishing rights without restriction.
License:
Open Access Journal - the journal offers free, immediate, and unrestricted access to peer-reviewed research and scholarly work, due SMTCT supports to increase the visibility, accessibility and reputation of the researchers, regardless of geography and their budgets. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author.













.png)















