Antagonism and plant growth promoting traits of actinomycetes isolated from the rhizosphere of halophyte Atriplex halimus L.
DOI:
https://doi.org/10.55779/nsb15111437Keywords:
actinomycetes, antagonism, Atriplex halimus, PGPR, rhizospheric soilAbstract
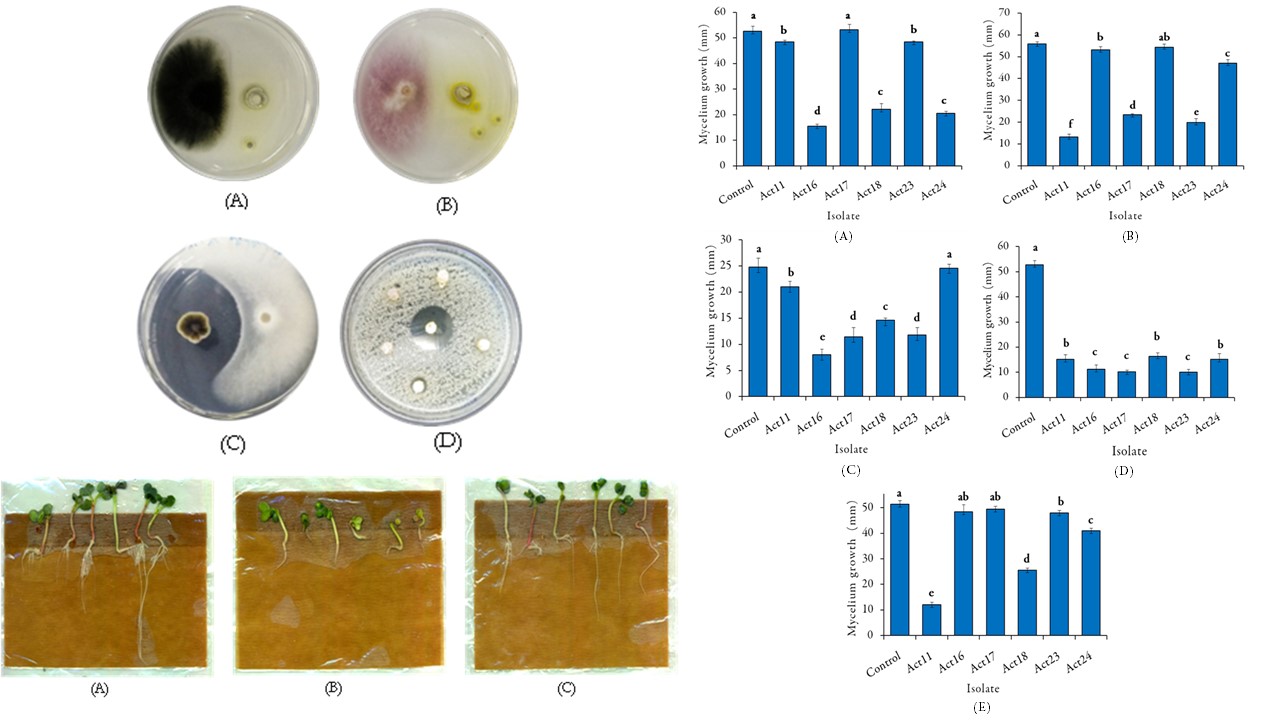
Biocontrol is considered as an effective alternative to the application of agrochemicals, which are harmful to the environment, human, and animal health. In this study, twenty-six strains of actinomycetes were isolated from rhizospheric arid soil of the halophyte Atriplex halimus L. ῾Guettaf’ in Biskra province, Algeria. The six isolates that have inhibited at least three phytopathogenic fungi among the five tested (Fusarium oxysporum, Alternaria alternata, Fusarium solani, Aspergillus flavus and Botrytis cinerea) were selected, and have been tested in vitro against phytopathogenic bacteria (Pectobacterium carotovorum and Streptomyces scabies). They were also evaluated for their ability to hydrolyze phosphate, elaborate siderophores, produce indole-3-acetic acid (IAA), and to antagonize S. scabies in vivo (on radish seedlings). Based on the physicochemical analyses, soil samples were categorized as alkaline and extremely-saline. The antagonism results revealed varying antifungal potential among the selected isolates (Act11, Act16, Act17, Act18, Act23 and Act24), about 50% were able to inhibit the growth of F. solani and A. flavus, followed by 33.33% of those having antagonized F. oxysporum, while A. alternata was found to be the most sensitive. Only Act18 has antagonized S. scabies in vitro with an inhibition diameter zone of 19 ± 0.41 mm. However, in vivo trials showed that four isolates have counteracted S. scabies. Among them, Act18 and Act24 have significantly and positively affected the root surface (P = 0.0062) and prevented common scab. IAA was detected in all selected isolates with Act24 being the highest producer (77.45 μg mL−1). Additionally, degradation ability revealed that four isolates were able to hydrolyze phosphate while three exhibited the capacity of elaborating siderophores. The six isolates were assigned to Streptomyces genius according to their morphological, physiological and chemotaxonomical traits. Based on this study, Streptomyces sp. Act18 and Streptomyces sp. Act24 that tolerate 7.5% NaCl concentration, prevent common scab and exhibit some plant growth attributes, may be considered as promising biocontrol agents to be applied in arid and saline soils.
Metrics
References
Ali A, Kurnia N, Asrini Nurani Ulfah A, Damayanti P, Rante H, Jumadi O (2021). Diversity of endophytic actinomycetes producing indole-3-acetic acid and in vitro evaluation of plant growth-promoting activity on brassica oleracea L. Tropical Agricultural Science 44(2):275-292. http://dx.doi.org/10.47836/pjtas.44.2.02
Alibrandi P, Lo Monaco N, Calevo J, Voyron S, Puglia AM, Cardinale M, Perotto S (2021). Plant growth promoting potential of bacterial endophytes from three terrestrial mediterranean orchid species. Plant Biosystems-An International Journal Dealing with all Aspects of Plant Biology 155(6):1153-1164. https://doi.org/10.1080/11263504.2020.1829731
Aouar L, Boukelloul I, Benadjila A (2020). Identification of antagonistic Streptomyces strains isolated from Algerian Saharan soils and their plant growth promoting properties. Biodiversitas Journal of Biological Diversity 21(12):5672-5683. http://dx.doi.org/10.13057/biodiv/d211212
Aouar L, Boukelloul I, Benadjila A (2021). PGPR traits of rhizospheric Nocardiopsis strains isolated from Algerian soils. In: Ksibo M, Ghorbal A, Chakraborty S, Chaminé SI, Barbieri M, Guerriero G, … Seffen M (Eds). Proceedings of 2nd Euro-Mediterranean Conference for Environmental Integration. Springer, Cham, Switzerland pp 1293-1300.
Aouar L, Lerat S, Ouffroukh A, Boulahrouf A, Beaulieu C (2012). Taxonomic identification of rhizospheric actinobacteria isolated from Algerian semi-arid soil exhibiting antagonistic activities against plant fungal Pathogens. Canadian Journal of Plant Pathology 34(2):165-176. https://doi.org/10.1080/07060661.2012.681396
Arora NK, Verma M (2017). Modified microplate method for rapid and efficient estimation of siderophore produced by bacteria. 3 Biotech 7:381. http://dx.doi.org/10.1007/s13205-017-1008-y
Ashwini M, Doddamani LC, Babar SR (2018). Characterization and evaluation of actinomycete isolates for traits associated with plant growth promotion. Journal of Pharmacognosy and Phytochemistry 7(6):1177-1180.
Beaudoin N, Isayenka I, Ducharme A, Massie S, Gagnon A, Hogue R, … Michaud D (2021). Habituation to thaxtomin A increases resistance to common scab in ‘Russet Burbank’ potato. PLoS One 16(6):e0253414. http://dx.doi.org/10.1371/journal.pone.0253414
Benadjila A, Zamoum M, Aouar L, Zitouni A, Goudjal Y (2022). Optimization of cultural conditions using response surface methodology and modeling of indole-3-acetic acid production by Saccharothrix texasensis MB15. Biocatalysis and Agricultural Biotechnology 39:102271. http://dx.doi.org/10.1016/j.bcab.2021.102271
Bona E, Massa N, Toumatia O, Novello G, Cesaro P, Todeschini V, … Gamalero E (2021). Climatic zone and soil properties determine the biodiversity of the soil bacterial communities associated to native plants from desert areas of North-Central Algeria. Microorganisms 9(7):1359. http://dx.doi.org/10.3390/microorganisms9071359
Chaudhary DR (2022). Halophytes of semi-arid areas: resources for mitigation of climate change. In: Poshiwa X, Ravindra Chary G (Eds). Climate Change Adaptations in Dryland Agriculture in Semi-Arid Areas. Springer, Singapore pp 93-109. https://doi.org/10.1007/978-981-16-7861-5_7
De Angelis G, Simonetti G, Chronopoulou L, Orekhova A, Badiali C, Petruccelli V, … Palocci C (2022). A novel approach to control Botrytis cinerea fungal infections: uptake and biological activity of antifungals encapsulated in nanoparticle based vectors. Scientific Reports 12:7989. http://dx.doi.org/10.1038/s41598-022-11533-w
Dif G, Belaouni HA, Yekkour A, Goudjal Y, Djemouai N, Peňázová E, … Zitouni A (2022). Performance of halotolerant bacteria associated with Sahara-inhabiting halophytes Atriplex halimus L. and Lygeum spartum L. ameliorate tomato plant growth and tolerance to saline stress: from selective isolation to genomic analysis of potential determinants. World Journal of Microbiology and Biotechnology 38:16. http://dx.doi.org/10.1007/s11274-021-03203-2
Ding LJ, Cui HL, Nie SA, Long XE, Duan GL, Zhu YG (2019). Microbiomes inhabiting rice roots and rhizosphere. FEMS Microbiology and Ecology 95(5):fiz040. https://doi.org/10.1093/femsec/fiz040
Djebaili R, Pellegrini M, Smati M, Del Gallo M, Kitouni M (2020). Actinomycete strains isolated from saline soils: Plant-growth-promoting traits and inoculation effects on solanum lycopersicum. Sustainability 12(11):4617. http://dx.doi.org/10.3390/su12114617
Ebrahimi-Zarandi M, Bonjar GHS, Riseh RS, El-Shetehy M, Saadoun I, Barka EA (2021). Exploring Two Streptomyces Species to control Rhizoctonia solani in Tomato. Agronomy 11(7):1384. http://dx.doi.org/10.3390/agronomy11071384
Goodfellow M, Kampfer P, Busse HJ, Trujillo ME, Suzuki KI, Ludwig W, Whitman WB (2012). Bergey’s Manual of Systematic Bacteriology. New York, Springer.
Hasegawa T, Takizawa M, Tanida S (1983). A rapid analysis for chemical grouping of aerobic actinomycetes. The Journal of General and Applied Microbiology 29(4):319-322. https://doi.org/10.2323/jgam.29.319
Kang MK, Lee GS, Lee MS, Choi HJ, Park DH (2022). Biocontrol efficacy of antagonistic and endophytic Streptomyces sp. against common scab disease. Journal of Plant Diseases and Protection 129(5):1115-1124. https://doi.org/10.1007/s41348-022-00602-x
Khenaka K, Canfora L, Benedetti A, Leulmi N, Boulahrouf A (2019). Effect of Capsicum annuum cultivated in sub-alkaline soil on bacterial community and activities of cultivable plant growth promoting bacteria under field conditions. Archives of Agronomy and Soil Science 65(10):1417-1430. http://dx.doi.org/10.1080/03650340.2019.1566711
Le KD, Yu NH, Park AR, Park DJ, Kim CJ, Kim JC (2022). Streptomyces sp. AN090126 as a Biocontrol Agent against Bacterial and Fungal Plant Diseases. Microorganisms 10(4):791. http://dx.doi.org/10.3390/microorganisms10040791
Lebourgeois F, Piedallu C (2005). Appréhender le niveau de sécheresse dans le cadre des études stationnelles et de la gestion forestière à partir d’indices bioclimatiques [Understanding the level of drought in the context of station studies and forest management based on bioclimatic indices]. Revue Forestière Française 57(4):331-356.
Lee JY, Hwang BK (2002). Diversity of antifungal actinomycetes in various vegetative soils of Korea. Canadian Journal of Microbiology 48(5):407-417. http://dx.doi.org/10.1139/w02-025
Lee SM, Kong HG, Song GC, Ryu CM (2021). Disruption of Firmicutes and Actinobacteria abundance in tomato rhizosphere causes the incidence of bacterial wilt disease. The ISME Journal 15:330-347. http://dx.doi.org/10.1038/s41396-020-00785-x
Legault GS, Lerat S, Nicolas P, Beaulieu C (2011). Tryptophan regulates thaxtomin A and indole-3 acetic acid production in Streptomyces scabiei and modifies its interactions with radish seedlings. Phytopathology 101(9):1045-1051. https://doi.org/10.1094/PHYTO-03-11-0064
Nonthakaew N, Panbangred W, Songnuan W, Intra B (2022). Plant growth-promoting properties of Streptomyces spp. isolates and their impact on mung bean plantlets' rhizosphere microbiome. Frontiers in Microbiology 13:967415. http://dx.doi.org/10.3389/fmicb.2022.967415
Oleńska E, Małek W, Wójcik M, Swiecicka I, Thijs S, Vangronsveld J (2020). Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Science of the Total Environment 743:140682. http://dx.doi.org/10.1016/j.scitotenv.2020.140682
Parasuraman P, Pattnaik SS, Busi S, Marraiki N, Elgorban AM, Syed A (2022). Isolation and characterization of plant growth promoting rhizobacteria and their biocontrol efficacy against phytopathogens of tomato (Solanum lycopersicum L.). Plant Biosystems 156(1):164-170. http://dx.doi.org/10.1080/11263504.2020.1845842
Putrie RFW, Aryantha INP, Iriawati I, Antonius S (2020). Diversity of endophytic and rhizosphere bacteria from pineapple (Ananas comosus) plant in semi-arid ecosystem. Biodiversitas Journal of Biological Diversity 21(7):3084-3093. http://dx.doi.org/10.13057/biodiv/d210728
Qadir SA, Atalan E (2019). Biodiversity of Streptomyces of from soil samples of Halapja, Iraq. Yüzüncü Yıl Üniversitesi Fen Bilimleri Enstitüsü Dergisi 24(2):72-87.
Qin S, Miao Q, Feng WW, Wang Y, Zhu X, Xing K, Jiang JH (2015). Biodiversity and plant growth-promoting traits of culturable endophytic actinobacteria associated with Jatropha curcas L. growing in Panxi dry-hot valley soil. Applied Soil Ecology 93:47-55. http://dx.doi.org/10.1016/j.apsoil.2015.04.004
Rana KL, Kour D, Yadav AN (2019). Endophytic microbiomes: biodiversity, ecological significance and biotechnological applications. Research Journal of Biotechnology 14(10):142-162.
Richards LA (1954). Diagnosis and improvement of saline and alkali soils. Washington, USA.
Rios-Muñiz DE, Evangelista-Martínez Z (2022). Antifungal activity of Streptomyces sp. CACIS-2.15CA, as a potential biocontrol agent, against some soil-borne fungi. Egyptian Journal of Biological Pest Control 32:130. http://dx.doi.org/10.1186/s41938-022-00630-7
Shirling ET, Gottlieb D (1966). Methods for characterization of Streptomyces species. International Journal of Systematic Bacteriology 16(3):313-340.
Sreevidya M, Gopalakrishnan S, Kudapa H, Varshney RK (2016). Exploring plant growth-promotion actinomycetes from vermicompost and rhizosphere soil for yield enhancement in chickpea. Brazilian Journal Microbiology 47(1):85-95. http://dx.doi.org/10.1016/j.bjm.2015.11.030
Staneck JL, Roberts GD. (1974). Simplified approach to identificationof aerobic actinomycetes by thin-layer chromatography. Applied Microbiology 28(2):226-231.
Suárez-Moreno ZR, Vinchira-Villarraga DM, Vergara-Morales DI, Castellanos L, Ramos FA, Guarnaccia C, Moreno-Sarmiento N (2019). Plant-growth promotion and biocontrol properties of three Streptomyces spp. isolates to control bacterial rice pathogens. Frontiers in Microbiology 10:290. http://dx.doi.org/10.3389/fmicb.2019.00290
Sudiana IM, Putri A, Napitupulu TP, Purnaningsih I, Kanti A (2020). Growth inhibition of Fusarium solani and F. oxysporum by Streptomycessasae TG01, and its ability to solubilize insoluble phosphate. Biodiversitas Journal of Biological Diversity 21(2):429-435. http://dx.doi.org/10.13057/biodiv/d210201
Tahtamouni ME, Khresat SE, Lucero M, Sigala J, UNC A. (2016). Diversity of endophytes across the soil-plant continuum for Atriplex spp. in arid environments. Journal of Arid Land 8:241-253. http://dx.doi.org/10.1007/s40333-015-0061-9
Vurukonda SS, Giovanadri D, Stefani E (2021). Growth promotion and biocontrol activity of endophytic Streptomyces spp. Prime Archives in Molecular Sciences, 2nd Edition 1:1-55. http://hdl.handle.net/11380/1248582
Wang M, Xue J, Ma J, Feng X, Ying H, Xu H. 2020. Streptomyces lydicus M01 regulates soil microbial community and alleviates foliar disease caused by Alternaria alternata on cucumbers. Frontiers in Microbiology 11:942. http://dx.doi.org/10.3389/fmicb.2020.00942
Warrad M, Hassan YM, Mohamed MSM , Hagagy N, Al-Maghrabi OA, Selim S, … Abd Elgawad H (2020). A bioactive fraction from Streptomyces sp. enhances maize tolerance against drought stress. Journal of Microbiology and Biotechnology 30(8):1156-1168. http://dx.doi.org/10.4014/jmb.2003.03034
Williams ST, Cross T (1971). Actinomycetes. In: Booth C (Ed). Method in Microbiology. Academic Press, London pp 295-334.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Inas BOUKELLOUL, Lamia AOUAR, Mohamed CHEKARA BOUZIANI, Amar ZELLAGUI, Mouna DERDOUR, Youcef NECIB

This work is licensed under a Creative Commons Attribution 4.0 International License.
Papers published in Notulae Scientia Biologicae are Open-Access, distributed under the terms and conditions of the Creative Commons Attribution License.
© Articles by the authors; licensee SMTCT, Cluj-Napoca, Romania. The journal allows the author(s) to hold the copyright/to retain publishing rights without restriction.
License:
Open Access Journal - the journal offers free, immediate, and unrestricted access to peer-reviewed research and scholarly work, due SMTCT supports to increase the visibility, accessibility and reputation of the researchers, regardless of geography and their budgets. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author.













.png)















