Enhanced production of lupeol through elicitation in in vitro shoot cultures of snake grass (Clinacanthus nutans)
DOI:
https://doi.org/10.55779/nsb14411195Keywords:
chitosan, Clinacanthus nutans, elicitors, HPTLC, lupeol; methyl jasmonate, yeast extractAbstract
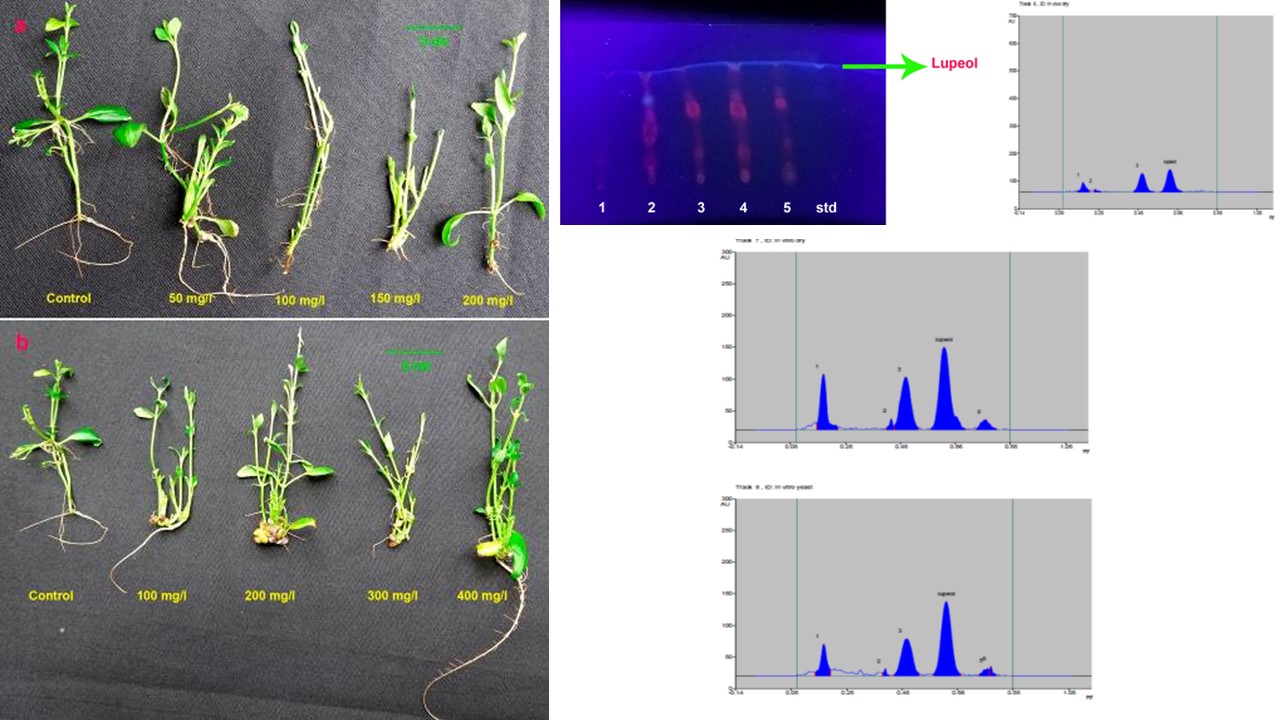
Clinacanthus nutans (Acanthaceae), generally known as ‘snake grass’, has diverse uses in customary system of herbal medicine. The species is endowed with various bioactive compounds exhibiting extensive pharmacological properties. The present investigation focused on elicitor-intervened in vitro shoot biomass cultivation and scale-up production of the anti-cancerous compound ‘lupeol’, one of the foremost constituents in this species. For the augmented production of lupeol, the shoot cultures were elicited with various concentrations of yeast extract (YE), chitosan and methyl jasmonate (MeJA). Maximum shoot biomass yield and production of lupeol was detected in MS medium supplemented with 1.0 mgl-1 BA and 400 mgl-1 YE. The petroleum ether extracts of selected samples upon TLC analysis proved Rf values corresponding to lupeol. HPTLC analysis revealed that the sample treated with YE displayed relatively higher amount (975.50 ng) of lupeol than the in vivo plant (713.69 ng). Hence the in vitro shoot culture system with elicitor (YE) treatment propose an appropriate method for the elevated synthesis of lupeol which can be scaled up via bio-reactor technology in doing so profiting the pharmaceutical appliances.
Metrics
References
Bong FJ, Chear NJY, Ramanathan S, Mohana-Kumaran N, Subramaniam S, Chew BL (2021). The development of callus and cell suspension cultures of Sabah Snake Grass (Clinnacanthus nutans) for the production of flavonoids and phenolics. Biocatalysts and Agricultural Biotechnology 33. https://doi.org/10.1016/j.bcab.2021.101977
Chen B, Zhang JZC, Xiao Y (2015). The rapid propagation technique of the medicinal plant Clinacanthus nutans by tissue culture. New York Science Journal 8:23-27. https://doi.org/10.7537/j.issn.1554-0200
Dampawan P, Huntrakul C, Reutrakul V, Raston CL, White AH (1977). Constituents of Clinacanthus nutans and crystal structure of Lup-20(29)-ene-3-one. Journal of the Science Society of Thailand 3:14-26. https://doi.org/10.2306/scienceasia1513-1874.1977.03.014
George EF, Hall MA, De Klerk G (2007). Plant propagation by tissue culture. Vol. I, 3rd edition. Springer.
Gras MCS, Calvo MC (1996). Micropropagation of Lavendula latifolia through nodal bud culture of mature plants. Plant Cell Tissue and Organ Culture 45:259-261. https://doi.org/10.1007/BF00043639
Gunasekaran U (2014). Callus induction and plant regeneration studies of Clinacanthus nutans. Bachelor Thesis, Tunku Abdul Rahman University, Kuala Lumpur, Malaysia.
Haida Z, Hakiman M (2019). A review of therapeutic potentials of Clinacanthus nutans as source for alternative medicines. Sains Malaysiana 48(12):2683-2691. http://dx.doi.org/10.17576/jsm-2019-4812-09.
Haida Z, Nakasha JJ, Hakiman M (2020). In vitro responses of pant growth factors on growth, yield, phenolics content and antioxidant activities of Clinacanthus nutans (Sabah Snake Grass). Plants 9:1030. https://doi.org/10.3390/plants9081030
Jain PS, Bari SB (2010). Isolation of lupeol, stigmasterol and campesterol from petroleum ether extract of woody stem of Wrightia tinctoria. Asian Journal of Plant Sciences 9:163-167. https://dx.doi.org/10.3923/ajps.2010.163.167.
Jalili Marandi R, Naseri L, Mohseniazer M, Hajitagiloo R, Marhamati MR (2011) Investigation on interaction effect of benzyladanine and chitosan on in vitro proliferation of strawberry. Agricultural Biotechnology (Agricultural Research) 10:27-34.
Kanchanapoom K, Pimolthai P, Kanchananpoom K (2012). The Effect of chitosan on regeneration of lily (Lilium longiflorum Thunb. ‘Ester Lily’) from bulb scale explants cultured in vitro. Propagation of Ornamental Plants 12:127-129.
Liu X, Zhang X, Sun J (2007). Effects of cytokinins and elicitors on the production of hypericins and hyperforin metabolites in Hypericum sampsonii and Hypericum perforatum. Plant Growth Regulation 53:207-214. https://doi.org/10.1007/s10725-007-9220-0
Murashige, T, Skoog F (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiologia Plantarum 15:473-497. http://dx.doi.org/10.1111/j.1399-3054.1962.tb08052.x.
Naik PM, Al-Khayri JM (2016). Impact of abiotic elicitors on in vitro production of plant secondary metabolites: A review. Journal of Advanced Research in Biotechnology 1:7. http://dx.doi.org/10.15226/2475-4714/1/2/00102.
Namdeo AG (2007). Plant Cell elicitation for production of secondary metabolites a: A Review. Pharmacognosy Reviews 1:69-79.
Ng LY (2013). Establishment of axenic explants and callus culture of Clinacanthus nutans (rumputbelalaigajah). Bachelor, Project Report, Universiti Malaysia, Sarawak.
P’ng XW, Akowuah GA, Chin JH (2013). Evaluation of the sub-acute oral toxic effect of methanol extract of Clinacanthus nutans leaves in rats. Journal of Acute Disease 2:29-32. https://doi.org/10.1016/S2221-6189(13)60090-6
Radman R, Saez T, Bucke C, Keshavarz T (2003). Elicitation of plant and microbial systems. Biotechnology and Applied Biochemistry 37:91-102. https://doi.org/10.1042/ba20020118.
Shakya P, Marslin G, Siram K, Beerhues L, Franklin G (2017). Elicitation as a tool to improve the profiles of high-value secondary metabolites and pharmacological properties of Hypericum perforatum. Journal of Pharmacy and Pharmacology 71(1):70-82. https://doi.org/10.1111/jphp.12743
Shim SY, Aziana I, Khoo BY (2013). Perspective and insight on Clinacanthus nutans Lindau in traditional medicine. International Journal of Biological Macromolecules 14:7-9.
Sridhar TM, Aswath CR (2014). Review on medicinal plants propagation: a comprehensive study on role of natural organic extracts in tissue culture medium. American Journal of Plant Sciences 5:3073-3088. http://dx.doi.org/10.4236/ajps.2014.520324.
Sriwanthana B, Chavalittumrong P, Chompuk L (1996). Effect of Clinacanthus nutans on human cell-mediated immune response in vitro. Thai Journal of Pharmaceutical Sciences 20:261-267.
Tejasen P, Thongthaap T (1978). The antagonistic effect against cobra envenomization comparison of medicinal plants, corticosteroid and antivenine anti. Chiang Mai Medical Bulletin 17:159-168.
Teshima KI, Kaneko T, Ohtani K, Kasai R, Lhieochaiphant S, Picheansoonthan C, Yamasaki K (1997). C-glycosyl flavones from Clinacanthus nutans. Nature Medicine 51:557-560.
Teshima KI, Kaneko T, Ohtani K, Kasai R, Lhieochaiphant S, Picheansoonthan C, Yamasaki K (1998). Sulfur-containing glucosides from Clinacanthus nutans. Phytochemistry 48:831-835. https://doi.org/10.1016/S0031-9422(97)00956-4
Thongharb C, Tejasen P (1977). The effect of Slaed pang porn (Clinacanthus nutans) on Thailand cobra venom (Naja Naja Siamensis). Thai Journal of Pharmaceutical Sciences 2:1057-1063.
Tuntiwachwuttikul P, Pootaeng-on Y, Phansa P, Taylor WC (2004). Cerebrosides and a monoacylmonogalactosylglycerol from Clinacanthus nutans. Chemical and Pharmaceutical Bulletin (Tokyo) 52:27-32. https://doi.org/10.1248/cpb.52.27
Wanikiat P, Panthong A, Sujayanon P, Yoosook C, Rossi AG, Reutrakul V (2008). The anti-inflammatory effects and inhibition of neutrophil responsiveness by Barleria lupulina and Clinacanthus nutans. Journal of Ethnopharmacology 116:234-244. https://doi.org/10.1016/j.jep.2007.11.035
Downloads
Published
How to Cite
Issue
Section
License
Papers published in Notulae Scientia Biologicae are Open-Access, distributed under the terms and conditions of the Creative Commons Attribution License.
© Articles by the authors; licensee SMTCT, Cluj-Napoca, Romania. The journal allows the author(s) to hold the copyright/to retain publishing rights without restriction.
License:
Open Access Journal - the journal offers free, immediate, and unrestricted access to peer-reviewed research and scholarly work, due SMTCT supports to increase the visibility, accessibility and reputation of the researchers, regardless of geography and their budgets. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author.













.png)















