In vitro and in silico investigation of the antifungal activity of endophytic fungi against phytopathogenic fungi of tomato
DOI:
https://doi.org/10.15835/nsb14111050Keywords:
3HNR, biological control, endophytic microorganisms, molecular docking, phytopathogenic fungiAbstract
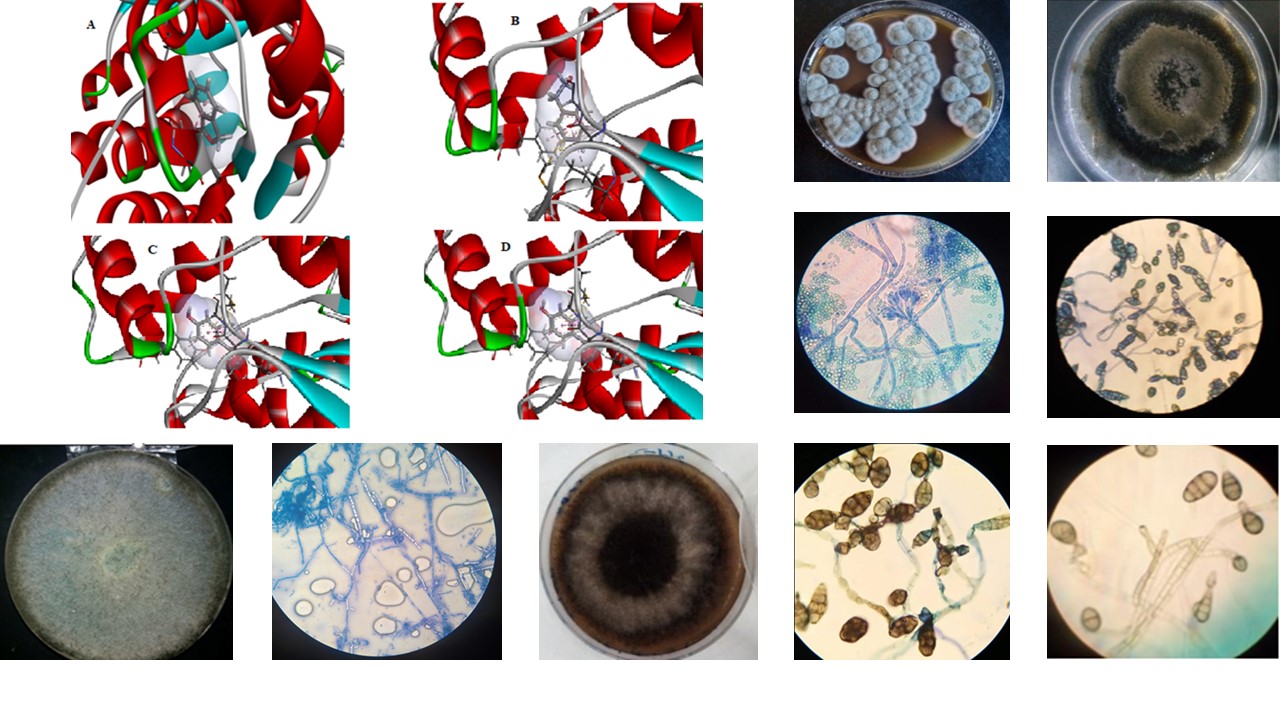
Plants are threatened by several diseases caused by phytopathogenic fungi. Melanin is an important pathogenicity factor in some fungal plant diseases. The enzyme 6,3,8-trihydroxynaphthalene reductase (3HNR) is implicated in the catalysis of the melanin biosynthesis in fungi. The chemical fungicide Phthalide acts by inhibiting this enzyme. But despite its efficacy, Phthalide can be detrimental to environmental health, hence the need to look for natural inhibitors to combat phytopathogenic fungi. This study aimed to screen the antifungal activity of some endophytic strains against phytopathogenic fungi of tomato. A total of 7 endophytic fungi were isolated and pre-identified from different parts of celery, parsley, mint, and coriander. On the other hand, five phytopathogenic fungal strains were isolated and pre-identified from tomatoes. The agar cylinder method showed that the endophytic fungi strains Fusarium and Trichoderma have significant inhibitory activity against four phytopathogenic fungi identified as Alternaria and Penicillium. Molecular docking was also used to study the inhibitory effect of some bioactive fungal compounds against the 3HNR enzyme. Drug-likeness and ADMET analyses were conducted on the selected chemicals to test their reliability and pharmaceutical efficacy. Phenylethyl alcohol interacts intensely with the binding site of the 3HNR receptor giving binding energy of -5.3 Kcal/mol, which is close to the co-crystallized ligand Phthalide. In addition, ADMET and pharmacokinetic analysis revealed that Phenylethyl alcohol verify the majority of the filters and pharmacokinetic properties necessary to select an effective antifungal molecule, including Lipinski’s and Veber’s rules.
Metrics
References
Bell AA, Wheeler MH (1986). Biosynthesis and functions of fungal melanins. Annual Review of Phytopathology 24(1):411-451.https://doi.org/10.1146/annurev.py.24.090186.002211
Belozerskaya TA, Gessler NN, Aver’yanov AA (2017). Melanin pigments of fungi. Fungal Metabolites Reference Series in Phytochemistry. Cham., Springer. https://doi.org/10.1007/978-3-319-25001-4_29
Benítez T, Rincón AM, Limón MC, Codon AC (2004). Biocontrol mechanisms of Trichoderma strains. International Microbiology 7(4):249-260.
Botton B, Breton A, Fèvre M, Guy PH, Larpent JP, Veau P (1990). Moisissures utiles et nuisibles. Importance industrielle. [Useful and harmful molds. Industrial importance]. Dunod (2ème Ed), collection: Biotechnologies, pp 512.
Butler MJ, Gardiner RB, Day AW (2005). Degradation of melanin or inhibition of its synthesis: are these a significant approach as a biological control of phytopathogenic fungi? Biological Control 32(2):326-336. https://doi.org/10.1016/j.biocontrol.2004.08.008
Chabasse D, Bouchara JP, de Gentile L, Brun S, Cimon B, Penn P (2002). Les moisissures à intérêt médical. [Molds of medical interest]. Cahier de formation N° 25. Bioforma 230:75014. https://doi.org/10.1016/S0338-9898(05)80232-X
Chaur T (1998). General mechanisms of action of microbial biocontrol agents. Plant Pathology Bulletin 7:155-166. http://dx.doi.org/10.6649%2fPPB.199812_7(4).0001
Cheng F, Li W, Zhou Y, Shen J, Wu Z, Liu G, Tang Y (2012). admetSAR: a comprehensive source and free tool for assessment of chemical ADMET properties. Journal of Chemical Information and Modeling. https://doi.org/10.1021/ci300367a
Cook RJ (1993). Making greater use of introduced microorganisms for biological control of plant pathogens. Annual Review of Phytopathology 31(1):53-80. https://doi.org/10.1146/annurev.py.31.090193.000413
Corbaz R (1990). Principes de phytopathologie et de lutte contre les maladies des plantes. [Principles of plant pathology and plant disease control]. PPUR Presses Polytechniques, pp 286.
Elad Y, Chet I, Boyle P, HenisY (1983). Parasitism of Trichoderma spp. on Rhizoctonia solani and Sclerotium rolfsii-scanning electron microscopy and fluorescence microscopy. Phytopathology 73(1):85-88. http://dx.doi.org/10.1094/Phyto-73-85
Ezra D, Hess WM, Strobel GA (2004). New endophytic isolates of Muscodor albus, a volatile-antibiotic-producing fungus. Microbiology150(12):4023-4031. https://doi.org/10.1099/mic.0.27334-0
Forli W, Halliday S, Belew R, Olson AJ (2012). AutoDock Version 4.2. Journal of Medicinal Chemistry 55(2):623-638. https://doi.org/10.1021/jm2005145
Huh EJ, Kim JW (2010). Consumer knowledge and attitude to spending on environment-friendly agricultural products. Korean Journal of Human Ecology 19(5):883-896. https://doi.org/10.5934/KJHE.2010.19.5.883
Iqbal Z, Khan MA, SharifM, Shah JH, urRehman MH, Javed K (2018). An automated detection and classification of citrus plant diseases using image processing techniques: A review. Computers and Electronics in Agriculture 153:12-32. https://doi.org/10.1016/j.compag.2018.07.032
Jacobson ES (2000). Pathogenic roles for fungal melanins. Clinical Microbiology Reviews 13(4):708-717. https://doi.org/10.1128/CMR.13.4.708
Khasa YP (2017). Microbes as biocontrol agents. In: Probiotics and Plant Health. pp 507-552. http://dx.doi.org/10.1007/978-981-10-3473-2_24
Kidd S, Halliday CL, Alexiou H, Ellis D (2016). Descriptions of medical fungi. (Vol. 3). David Ellis.
Kumar V, Haldar S, Pandey KK, Singh RP, Singh AK, Singh PC (2007). Cultural, morphological, pathogenic, and molecular variability amongst tomato isolates of Alternaria solani in India. World Journal of Microbiology and Biotechnology 24(7):1003-1009. https://doi.org/10.1007/s11274-007-9568-3
Lazarovits G, Turnbull A, Johnston-Monje D (2014). Plant health management: biological control of plant pathogens. http://dx.doi.org/10.1016/B978-0-444-52512-3.00177-7
Leslie JF SummerellBA (2008). The Fusarium laboratory manual. John Wiley & Sons. https://doi.org/10.1002/9780470278376
Madigan MT, Martinko JM, Parker J (1997). Brock biology of microorganisms. (Vol. 11). Upper Saddle River, NJ, Prentice Hall.
Nega A (2014). Review on concepts in biological control of plant pathogens. Journal of Biology, Agriculture and Healthcare 4(27):33-54.
Nosanchuk JD, Stark RE, Casadevall A (2015). Fungal melanin: what do we know about structure? Frontiers in Microbiology 6:1463. https://doi.org/10.3389/fmicb.2015.01463
O’Brien PA (2017). Biological control of plant diseases. Australasian Plant Pathology 46(4):293-304. https://doi.org/10.1007/s13313-017-0481-4
Pankhurst CE, Lynch JM (2005). Biocontrol of soil-borne plant diseases. Pp 133.
Rodrigues TTMS, Berbee ML, Simmons EG, Cardoso CR, Reis A, Maffia LA, Mizubuti ESG (2010). First report of Alternaria tomatophila and A. grandis causing early blight on tomato and potato in Brazil. New Disease Reports 22:28-28. http://dx.doi.org/10.5197/j.2044-0588.2010.022.028.
Sajeena A, Nair DS,SreepavanK (2020). Non-pathogenic Fusarium oxysporum as a biocontrol agent. Indian Phytopathology 73(2):177-183. https://doi.org/10.1007/s42360-020-00226-x
Silva Jr L, Carrion LL, von Groll A, Costa SS, Junqueira E, Ramo, DF, … Almeida da Silva PEA (2017). In vitro and in silico analysis of the efficiency of tetrahydropyridines as drug efflux inhibitors in Escherichia coli. International Journal of Antimicrobial Agents 49(3):308-314. https://doi.org/10.1016/j.ijantimicag.2016.11.024
Tannous J, Barda O, Luciano-Rosario D, Prusky DB, Sionov E, Keller NP. (2020). New insight into pathogenicity and secondary metabolism of the plant pathogen Penicillium expansum through deletion of the epigenetic reader SntB. Frontiers in Microbiology 11:610. https://doi.org/10.3389/fmicb.2020.00610 .
Thambugala KM, Daranagama DA, Phillips AJ, Kannangara SD, Promputtha I (2020). Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Frontiers in Cellular and Infection Microbiology10. https://doi.org/10.3389/fcimb.2020.604923
Usta C (2013). Microorganisms in biological pest control - a review (bacterial toxin application and effect of environmental factors). Current Progress in Biological Research 13:287-317. http://dx.doi.org/10.5772/55786
Weindling R (1934). Studies on a lethal principle effective in the parasitic action of Trichoderma lignorum on Rhizoctonia solani and other soil fungi. Phytopathology 24(11): 1153-1179.
Weindling R, Fawcett H (1936). Experiments in the control of Rhizoctonia damping-off of citrus seedlings. Hilgardia 10(1):1-16. http://dx.doi.org/10.3733/hilg.v10n01p001
Yang HS, Sohn HB, Chung YR (2002). Biological control of pythium damping-off of cucumber by Bacillus stearothermophilus YC4194. Proceedings of the Korean Society of Plant Pathology 2002 Conference. The Korean Society of Plant Pathology, pp 49-52.
Zerrog A (2011). Bioactive secondary metabolites of endophytic fungi isolated from Retama raetam (Forssk). Magister theme. University Ferhat Abbas - Sétif. Algeria.
Downloads
Published
How to Cite
Issue
Section
License
Papers published in Notulae Scientia Biologicae are Open-Access, distributed under the terms and conditions of the Creative Commons Attribution License.
© Articles by the authors; licensee SMTCT, Cluj-Napoca, Romania. The journal allows the author(s) to hold the copyright/to retain publishing rights without restriction.
License:
Open Access Journal - the journal offers free, immediate, and unrestricted access to peer-reviewed research and scholarly work, due SMTCT supports to increase the visibility, accessibility and reputation of the researchers, regardless of geography and their budgets. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author.













.png)















