Apomictic development during different flower development stages in Crataegus tanacetifolia (Lam.) Pers., endemic to Turkey
DOI:
https://doi.org/10.15835/nsb13210911Keywords:
apomixis, apospory, flowering time, pollination time, RosaceaseAbstract
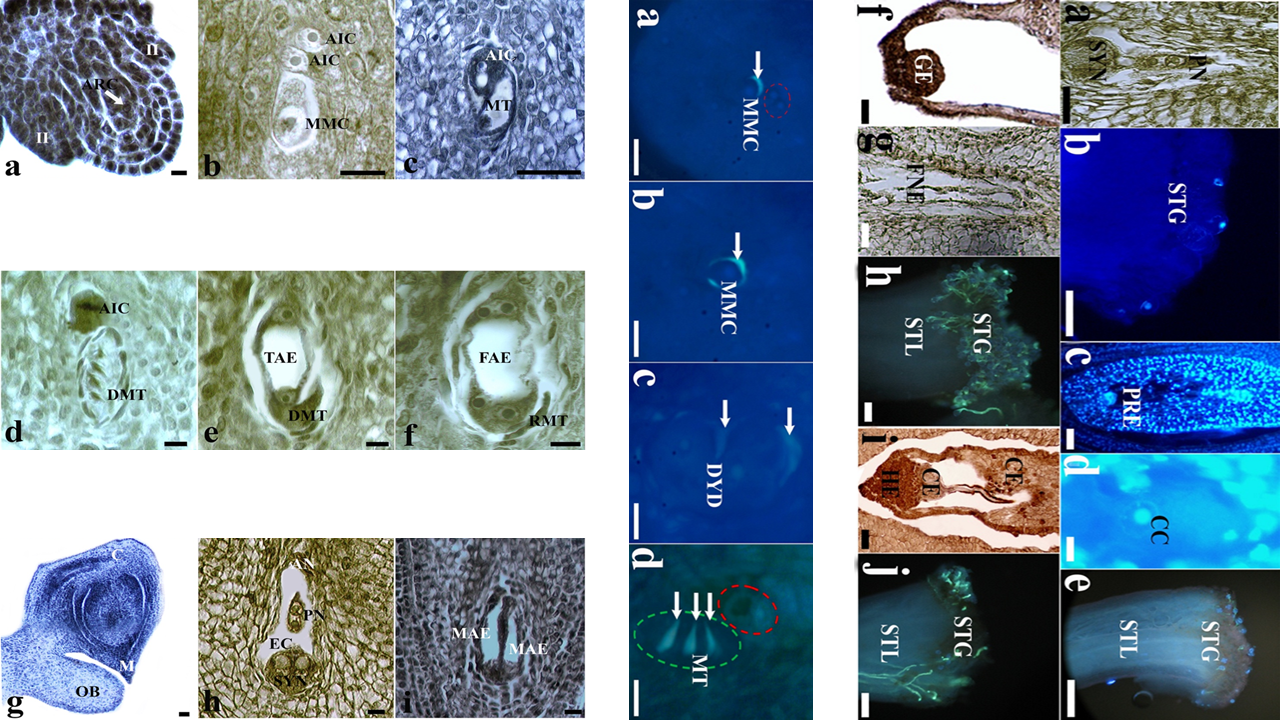
In this study, aposporic apomictic development and its relation to the different flower development stages were investigated by light and fluorescence microscopy in Crataegus tanacetifolia (Lam.) Pers. (Rosaceae). At pre-anthesis stage, aposporic initial cell differentiated at the somatic nucellus tissue shortly after the megaspore mother cell formation. The volume of aposporic initial cell increased during the generation of dyad and megaspore tetrad respectively by regular meiosis. At this stage, linear megaspore tetrad and vacuolated aposporic initial cell were located side by side into the same ovule. At anthesis stage, before pollination, four nucleated aposporic embryo sac was formed while sexual development came to end by atrophy of megaspores completely. At this stage, atrophied megaspores and two nucleated aposporic embryo sac were located side by side into the same ovule. At post-anthesis stage, pollination still had not begun and ovule contained only eight nucleated aposporic embryo sac. Mature aposporic embryo sac was composed of two synergid cells and one egg cell on the micropylar side, three antipodal cells on the chalazal side and a central cell with two polar nuclei in the middle of the sac. The absence of filiform apparatus in the synergid cells was quite remarkable. No callose accumulation around the aposporic initial cell was observed in any development stage. Pollination started shortly after the proembryo formation. Embryo and endosperm developed without fertilization due to the problems encountered in reaching pollen tubes to the ovary.
Metrics
References
Bicknell RA, Koltunow AM (2004). Understanding apomixis: recent advances and remaining conundrums. The Plant Cell 16:228-245. https://doi.org/10.1105/tpc.017921
Burson BL, Hussey MA, Actkinson JM, Shafer GS (2002). Effect of pollination time on the frequency of 2+ fertilization in apomictic Buffelgrass. Crop Science 42(4):1075-1080. https://doi.org/10.2135/cropsci2002.1075
Campbell CS, Evans RC, Morgan DR, Dickinson TA, Arsenault MP (2007). Phylogeny of subtribe Pyrinae (formerly the Maloideae, Rosaceae): limited resolution of a complex evolutionary history. Plant Systematic and Evolution 266(1):119-145. https://doi.org/10.1007/s00606-007-0545-y
Carman JG, Crane CF, Riera-Lizarazu O (1991). Comparative histology of cell walls during meiotic and apomeiotic megasporogenesis in two hexaploid Australasian Elymus species. Crop Science 31(6):1527-1532. https://doi.org/10.2135/cropsci1991.0011183X003100060029x
Drews GN, Koltunow AMG (2011). The female gametophyte. The Arabidopsis Book 9:e0155. https://doi.org/10.1199/tab.0155
Dusi DMA, Willemse MTM (1999). Activity and localization of sucrose synthase and invertase in ovules of sexual and apomictic Brachiaria decumbens. Protoplasma 208:173-185. https://doi.org/10.1007/BF01279088
Espinoza F, Pessino SC, Quarin CL, Valle EM (2002). Effect of pollination timing on the rate of apomictic reproduction revealed by RAPD markers in Paspalum notatum. Annals of Botany 89(2):165-170. https://doi.org/10.1093/aob/mcf024
Feder N, O’Brien TP (1968). Plant microtechnique: Some principles and new methods. American Journal of Botany 55(1):123-142. https://doi.org/10.1002/j.1537-2197.1968.tb06952.x
Fisher DB, Jensen WA, Ashton ME (1968). Histochemical studies of pollen: Storage pockets in the endoplasmic reticulum. Histochemie 13:169-182. https://doi.org/10.1007/BF00266578
Galla G, Barcaccia G, Schallau A, Molins MP, Bäumlein H, Sharbel TF (2011). The cytohistological basis of apospory in Hypericum perforatum L.. Sexual Plant Reproduction 24(1):47-61. https://doi.org/10.1007/s00497-010-0147-7
Hojsgaard D, Hörandl E (2019). The rise of apomixis in natural plant populations. Frontiers in Plant Science 10:358. https://doi.org/10.3389/fpls.2019.00358
Kenrick J, Knox RB (1985). Self-incompatibility in the nitrogen-fixing tree, Acacia retinodes: quantitative cytology of pollen tube growth. Theoretical and Applied Genetics 69(5-6):481-488.
Khan YJ, Choudhary R, Tyagi H, Singh AK (2015). Apomixis: The molecular perspectives and its utilization in crop breeding. Journal of AgriSearch 2(3):153-161.
Koltunow AM, Johnson SD, Bicknell RA (1998). Sexual and apomictic development in Hieracium. Sexual Plant Reproduction 11:213-230. https://doi.org/10.1007/s004970050144
Koscinska-Pajak M, Bednara J (2006). Unusual microtubular cytoskeleton of apomictic embryo sac of Chondrilla juncea L.. Protoplasma 227:87-93. https://doi.org/10.1007/s00709-006-0147-5
Kumar S (2017). Epigenetic control of apomixis: a new perspective of an old enigma. Advances in Plants and Agriculture Research 7(243):10-15406. https://doi.org/10.15406/apar.2017.07.00243
Leblanc O, Peel MD, Carman JG, Savidan Y (1995). Megasporogenesis and megagametogenesis in several Tripsacum species (Poaceae). American Journal of Botany 82:57-63. https://doi.org/10.1002/j.1537-2197.1995.tb15649.x
Lersten NR (2004). Flowering Plant Embryology. Blackwell Publishing Ames, USA.
Li JJ, Liu L, Ouyang YD, Yao JL (2011). Sexual reproduction development in apomictic Eulaliopsis binata (Poaceae). Genetics and Molecular Research 10(4):2326-2339. https://doi.org/10.4238/2011.October.5.3
Liu DD, Fang MJ, Dong QL, Hu DG, Zhou LJ, Sha GL, … Hao YJ (2014). Unreduced embryo sacs escape fertilization via a ‘female-late-on-date’ strategy to produce clonal seeds in apomictic crabapples. Scientia Horticulturae 167:76-83. https://doi.org/10.1016/j.scienta.2013.12.035
Musial K, Koscinska-Pajak M (2013). Ovules anatomy of selected apomictic taxa from Asteraceae family. Modern Phytomorphology 3:35-38.
Musial K, Koscinska-Pajak M, Antolec R, Joachimiak AJ (2015). Deposition of callose in young ovules of two Taraxacum species varying in the mode of reproduction. Protoplasma 252(1):135-144. https://doi.org/10.1007/s00709-014-0654-8
Naumova T, Nijs APM, Willemse MTM (1993). Quantitative analysis of aposporous parthenogenesis in Poa pratensis genotypes. Acta Botanica Neerlandia 42(3):299-312. https://doi.org/10.1111/j.1438-8677.1993.tb00707.x
Naumova T, Osadtchiy JV, Sharma VK, Dijkhuis P, Ramulu KS (1999). Apomixis in plants: structural and functional aspects of diplospory in Poa nemoralis and Poa palustris. Protoplasma 208:186-195. https://doi.org/10.1007/BF01279089
Naumova T, Willemse MTM (1995). Ultrastructural characterization of apospory in Panicum maximum. Sexual Plant Reproduction 8(4):192-204. https://doi.org/10.1007/BF00228937
Nogler GA (1984). Gametophytic Apomixis, Embryology of Angiosperms. Springer, New York.
Peel MD, Carman JG, Leblanc O (1997). Megasporocyte callose in apomictic buffelgrass, Kentucky bluegrass, Pennisetum squamulatum Fresen, Tripsacum L., and weeping lovegrass. Crop Science 37(3):724-732. https://doi.org/10.2135/cropsci1997.0011183X003700030006x
Priyadarshan PM (2019). Plant breeding: Classical to Modern. Springer, Singapore.
Robertson A, Rich TCG, Allen AM, Houston L, Roberts C, Bridle JR, … Hiscock SJ (2010). Hybridization and polyploidy as drivers of continuing evolution and speciation in Sorbus. Molecular Ecology 19(8):1675-1690. https://doi.org/10.1111/j.1365-294X.2010.04585.x
Roche D, Cong P, Chen Z, Hanna WW, Gustine DL, Sherwood RT, Ozias‐Akins P (1999). An apospory‐specific genomic region is conserved between Buffelgrass (Cenchrus ciliaris L.) and Pennisetum squamulatum Fresen. The Plant Journal 19(2):203-208. https://doi.org/10.1046/j.1365-313x.1999.00514.x
Sass JE (1958). Botanical microtechnique. Iowa State University Press Ames, USA.
Schweizer D (1976). Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma 58(4):307-324. https://doi.org/10.1007/BF00292840
Sharma R, Geeta R, Bhat V (2014). Asynchronous male/female gametophyte development in facultative apomictic plants of Cenchrus ciliaris (Poaceae). South African Journal of Botany 91:19-31. https://doi.org/10.1016/j.sajb.2013.10.012
Tang Q, Zang G, Zhao L, Cheng C, Dong Z, Gao C (2016). Embryological and genetic evidence of amphimixis and apomixis in Boehmeria tricuspis. Journal of Plant Biology 59(2):114-120. https://doi.org/10.1007/s12374-016-0518-1
Tucker MR, Koltunow AMG (2009). Sexual and asexual (apomictic) seed development in flowering plants: molecular, morphological and evolutionary relationships. Functional Plant Biology 36:490-504. https://doi.org/10.1071/FP09078
Tucker MR, Paech NA, Willemse MT, Koltunow AM (2001). Dynamics of callose deposition and β-1, 3-glucanase expression during reproductive events in sexual and apomictic Hieracium. Planta 212(4):487-498. https://doi.org/10.1007/s004250000445
Uzun M, Kaya A (2016). Ethnobotanical research of medicinal plants in Mihalgazi (Eskişehir, Turkey). Pharmaceutical Biology 54(12):2922-2932. https://doi.org/10.1080/13880209.2016.1194863
Yao JL, Zhou Y, Hu CG (2007). Apomixis in Eulaliopsis binata: characterization of reproductive mode and endosperm development. Sexual Plant Reproduction 20(3):151-158. https://doi.org/10.1007/s00497-007-0051-y
Zhang Y, Wang C, Wang K (2020). Research advances on plant synthetic apomixis. Chinese Science Bulletin 65(27):2998-3007. https://doi.org/10.1360/TB-2020-0209
Downloads
Published
How to Cite
Issue
Section
License
Papers published in Notulae Scientia Biologicae are Open-Access, distributed under the terms and conditions of the Creative Commons Attribution License.
© Articles by the authors; licensee SMTCT, Cluj-Napoca, Romania. The journal allows the author(s) to hold the copyright/to retain publishing rights without restriction.
License:
Open Access Journal - the journal offers free, immediate, and unrestricted access to peer-reviewed research and scholarly work, due SMTCT supports to increase the visibility, accessibility and reputation of the researchers, regardless of geography and their budgets. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author.













.png)















