Regeneration of Hemidesmus indicus (L.) R. Br. using in vitro nodes: an alternative method for efficient multiplication of shoots
DOI:
https://doi.org/10.15835/nsb13210831Keywords:
axillary shoots, clonal multiplication, cytokinins, medicinal plant, tissue cultureAbstract
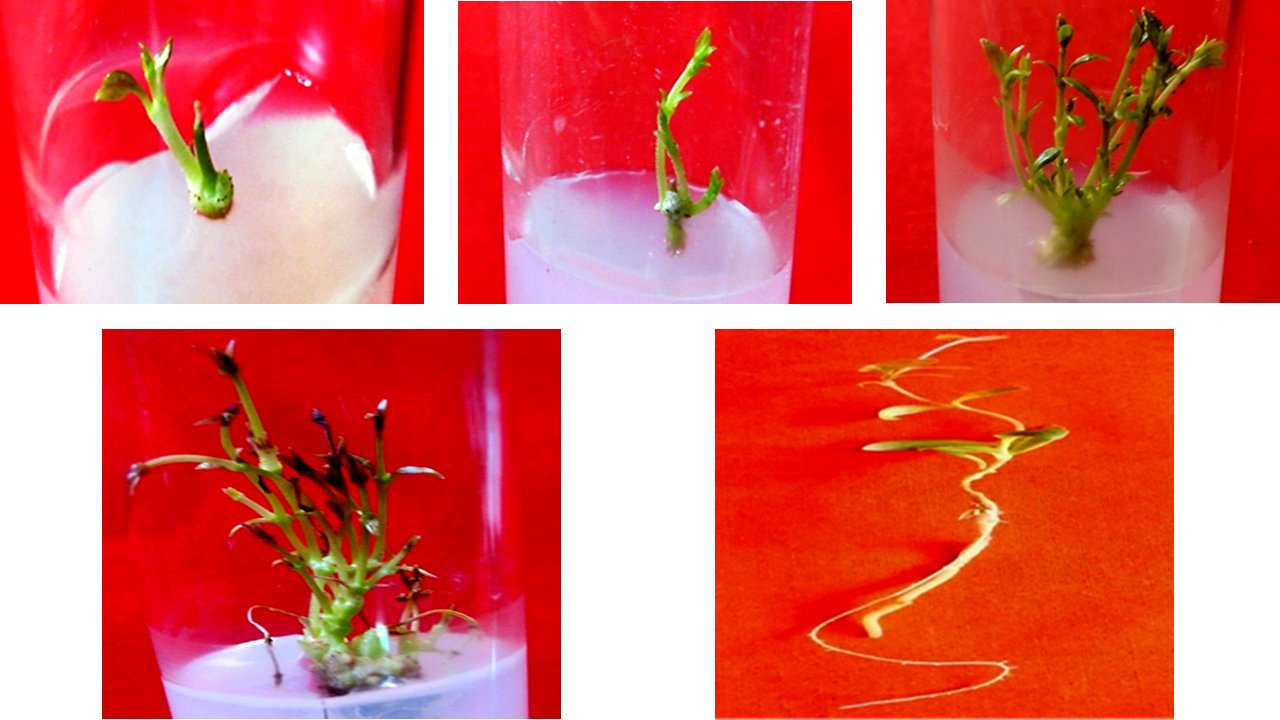
In vivo nodes of Hemidesmus indicus (L.) R. Br. induced healthy multiple shoots with branching in our earlier studies and thus in the present study, potency of in vitro nodes to regenerate shoots was evaluated. In vitro nodes were excised from eight-week-old shoots and placed in Murashige and Skoog’s (MS) medium fortified with sucrose (3%) and different concentrations of 6-benzyladenine (BA) and kinetin (Kn). After eight weeks, optimum of 5.42 ± 0.36 shoots with 100% response were regenerated in medium supplemented with BA (10 µM) and Kn (5 µM). These healthy shoots were placed in full, half and quarter strengths of liquid MS medium fortified with sucrose (1%) and α-naphthaleneacetic acid (NAA, 1-25 µM) for rooting. Among all the strengths of MS medium, full strength MS medium having 8 µM NAA formed maximum of 3.42 ± 0.55 roots (91.67% response) within four weeks. The protocol is in continuation with earlier study and it was confirmed that a single in vivo nodal explant can regenerate around 385 healthy elongated shoots within 4 months, which will help in mass-propagation of the species.
Metrics
References
Abebe Z, Mengesha A, Teressa A, Tefera W (2009). Efficient in vitro multiplication protocol for Vanilla planifolia using nodal explants in Ethiopia. African Journal of Biotechnology 8(24):6817-6821.
Anitha S, Pullaiah T (2002). In vitro propagation of Decalepis hamiltonii. Journal Tropical Medicinal Plants 3:227-232.
Anonymous (1997). The wealth of India, raw materials, vol. III, V and X. CSIR, New Delhi, India.
Baskaran P, Jayabalan N (2005). An efficient micropropagation system for Eclipta alba-a valuable medicinal herb. In Vitro Cellular and Developmental Biology-Plant 41:532-539. https://doi.org/10.1079/IVP2005667
Chatterjee RC, Bhattacharya BK (1955). A note on the isolation of β-sitosterol from Hemidesmus indicus. Journal of the Indian Chemical Society 32:485-486.
George EF (1996). Plant propagation by tissue culture. Part 2: In practice. Exegetics Ltd., Edington, UK.
George EF, Sharrington PD (1984). Plant propagation by tissue culture: Handbook and directory of commercial laboratories. Exegetics Ltd., Edington, UK.
Gupta MM, Verma RK, Misra LN (1992). Terpenoids from Hemidesmus indicus. Phytochemistry 31:4036-4037. https://doi.org/10.1016/S0031-9422(00)97582-4
Khadke S, Rani S, Awad V, Meti N, Singh E, Kuvalekar A, Harsulkar A (2013). An improved protocol for in vitro regeneration of Rubia cordifolia L. via organogenesis. International Journal of Plant, Animal and Environmental Sciences 3(4):61-69.
Loganathan K, Bai VN (2014). High frequency in vitro plantlet regeneration and antioxidant activity of Enicostema axillare (Lam.) Raynal ssp. littoralis (Blume) Raynal: An important medicinal plant. Asian Pacific Journal of Reproduction 3(3):241-248. https://doi.org/10.1016/S2305-0500(14)60033-6
Martin KP (2002). Rapid propagation of Holostemma ada-kodien Schult., a rare medicinal plant, through axillary bud multiplication and indirect organogenesis. Plant Cell Reports 21:112-117. https://doi.org/10.1007/s00299-002-0483-7
Misra N, Misra P, Datta SK, Mehrotra S (2003). Improvement in clonal propagation of Hemidesmus indicus R.Br. through adenine sulphate. Journal of Plant Biotechnology 5(4):239-244.
Mok DWS, Mok MC (2001). Cytokinin metabolism and action. Annual Review of Plant Physiology and Plant Molecular Biology 52:89-118. https://doi.org/10.1146/annurev.arplant.52.1.89
Murashige T, Skoog F (1962). A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiologia Plantarum 15:473-497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nadkarni AN (1989). Hemidesmus indicus R.Br. In: Nadkarni KM (Ed). Indian Materia Medica. Vol. 1. Popular Book Depot, Bombay, India pp 619-622.
Nagahatenna DSK, Peiris SE (2007). In vitro propagation of Hemidesmus indicus (L.) R. Br. (Iramusu) through nodal culture. Tropical Agricultural Research 19:181-192.
Nagarajan S, Rao LJ (2003). Determination of 2-hydroxy-4-methoxy benzaldehyde in roots of Decalepis hamiltonii (Wight & Arn.) and Hemidesmus indicus R.Br. Journal of AOAC International 86(3):564-567.
Pathak A, Joshi A (2017). Indirect organogenesis from leaf explants of Hemidesmus indicus (L.) R. Br.: An important medicinal plant. Plant Biosystems 151(1):1-5. https://doi.org/10.1080/11263504.2015.1108938
Pathak A, Joshi A (2018). Soma clonal variation. In: Prasad BD, Sahni S, Kumar P, Siddiqui WM (Eds). Plant Biotechnology. Vol. 1: Principles, Techniques and Applications. Apple Academic Press (CRC Press), New York, USA pp 185-213. https://doi.org/10.1201/9781315213743
Pathak A, Joshi A, Shrivastava N, Sharma P (2017). Regeneration and chemical profiling in Hemidesmus indicus (L.) R. Br. South African Journal 0f Botany 173:413-420. https://doi.org/10.1016/j.sajb.2017.09.022
Patnaik J, Debata BK (1996). Micropropagation of Hemidesmus indicus (L.) R.Br. through axillary bud culture. Plant Cell Reports 15:427-430. https://doi.org/10.1007/BF00232069
Planning Commission (2000). Report of the task force on conservation and sustainable use of medicinal plants. Government of India, New Delhi.
Puri HS (2003). Rasayana: Ayurvedic herbs for longevity and rejuvenation. Taylor and Francis, London and New York. https://doi.org/10.1201/b12602
Riov J, Yang S (1989). Ethylene and auxin-ethylene interaction in adventitious root formation in mung bean (Vigna radiata) cuttings. Journal of Plant Growth Regulation 8(2):131-141. https://doi.org/10.1007/BF02025280
Saha S, Mukhopadhyay MJ, Mukhopadhyay S (2003). In vitro clonal propagation through bud culture of Hemidesmus indicus (L.) R. Br: an important medicinal herb. Journal of Plant Biochemistry and Biotechnology 12:61-64. https://doi.org/10.1007/BF03263162
Sharma PK, Dhyani SK, Shankar V (1979). Some useful and medicinal plants of the district Dehradun and Siwalik. Journal of Scientific Research in Plant Medica 1:17-43.
Shekhawat MS, Manokari M (2016). In vitro regeneration frequency, micro-morphological studies and ex vitro rooting of Hemidesmus indicus (L.) R. Br.: a multipotent endangered climber. Indian Journal of Plant Physiology 21(2):151-160. https://doi.org/10.1007/s40502-016-0216-5
Sreekumar S, Seeni S, Pushpangadan P (2000). Micropropagation of Hemidesmus indicus for cultivation and production of 2-hydroxy-4-methoxy benzaldehyde. Plant Cell, Tissue and Organ Culture 62(3):211-218. https://doi.org/10.1023/A:1006486817203
Subramanian SS, Nair AGR (1968). Flavonoids of some Asclepiadaceous plants. Phytochemistry 7:1703-1704. https://doi.org/10.1016/S0031-9422(00)88630-6
Swamy MK, Balasubramanya S, Anuradha M (2010). In vitro multiplication of Pogostemon cablin Benth. through direct regeneration. African Journal of Biotechnology 9(14):2069-2075.
Taiz L, Zeiger E (2003). Auxins. In: Plant Physiology. Macmillan Publishing Co., New York, USA pp 623.
Thiyagarajan M, Venkatachalam P (2012). Large scale in vitro propagation of Stevia rebaudiana (Bert.) for commercial application: Pharmaceutically important and antidiabetic medicinal herb. Industrial Crops and Products 37:111-117. https://doi.org/10.1016/j.indcrop.2011.10.037
Thomas TD, Philip B (2005). Thidiazuron-induced high-frequency shoot organogenesis from leaf-derived callus of a medicinal climber, Tylophora indica (Burm. F.) Merrill. In Vitro Cellular and Developmental Biology-Plant 41:124-128. https://doi.org/10.1079/IVP2004575
Ved DK, Goraya GS (2007). Demand and supply of medicinal plants in India. NMPB, New Delhi and FRLHT, Bangalore, India.
Downloads
Published
How to Cite
Issue
Section
License
Papers published in Notulae Scientia Biologicae are Open-Access, distributed under the terms and conditions of the Creative Commons Attribution License.
© Articles by the authors; licensee SMTCT, Cluj-Napoca, Romania. The journal allows the author(s) to hold the copyright/to retain publishing rights without restriction.
License:
Open Access Journal - the journal offers free, immediate, and unrestricted access to peer-reviewed research and scholarly work, due SMTCT supports to increase the visibility, accessibility and reputation of the researchers, regardless of geography and their budgets. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author.













.png)















