Callogenesis in Cicer arietinum and identification of a genotype resistant to Ascochyta rabiei
DOI:
https://doi.org/10.15835/nsb12310788Keywords:
chickpea; histology; hormone balance; legume: pathogenAbstract
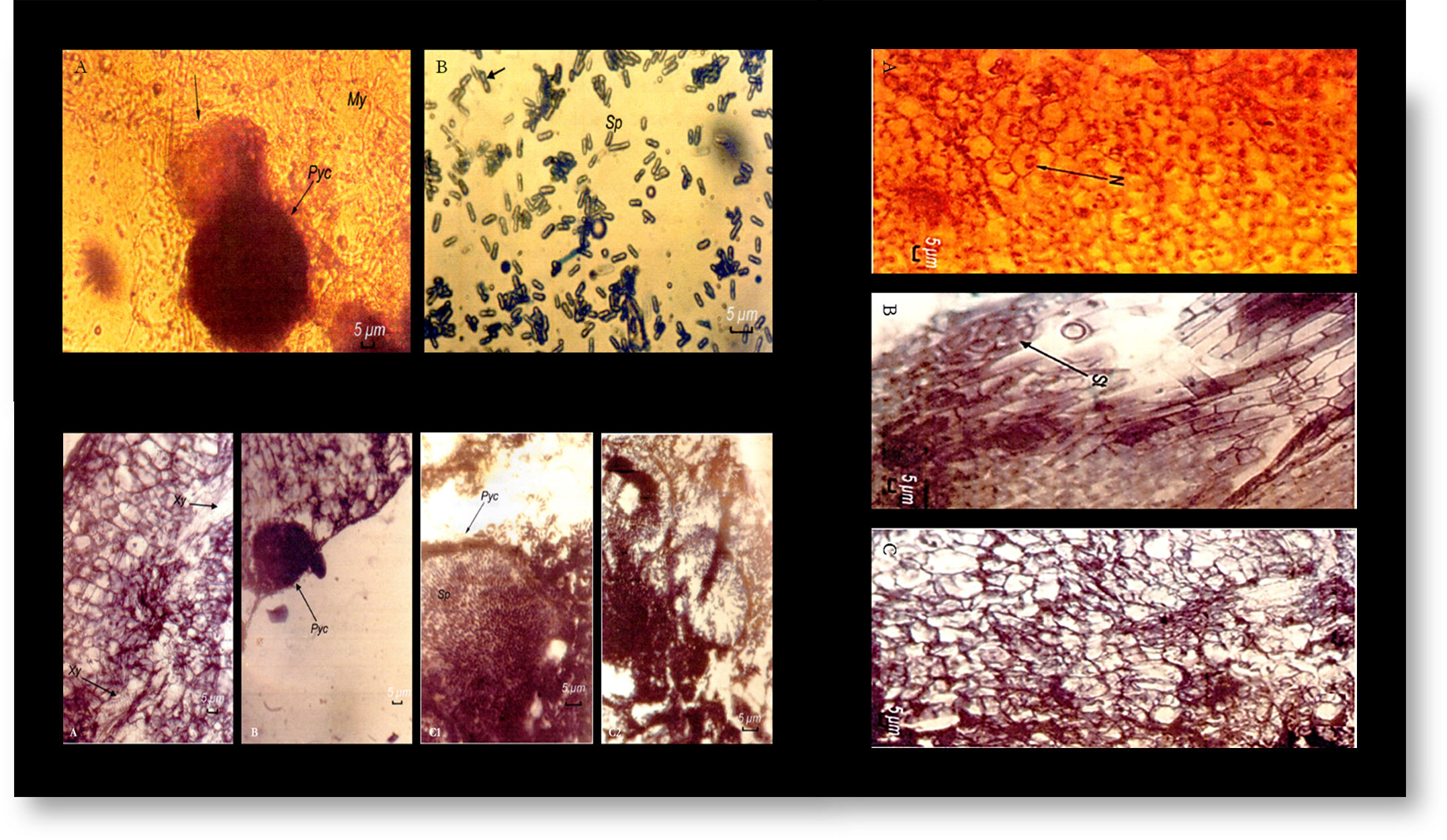
The chickpea (Cicer arietinum) is one of the leguminous species most appreciated by consumers in the Mediterranean basin, while being an important source of protein. Nevertheless, its crop yields are greatly limited by several biotic and abiotic stresses, the main one being Ascochyta rabiei, the causal agent of anthracnose. As traditional breeding methods have proved to be ineffective in controlling this pathogen, resorting to biotechnological methods is necessary. Therefore, in this study, the callogenic capacity of stem and leaflet explants from three genotypes of chickpea, namely ‘FLIP 84-92 C’, ‘ILC 32-97’, and ‘ILC 263’, cultured on Murashige and Skoog (MS) medium with different hormonal balances of auxins (indole-3-acetic acid [IAA] and 2,4-dichlorophenoxyacetic acid [2,4-D]) and cytokinin (kinetin), was determined. For all the genotypes, high percentages of callogenesis were recorded in the different explants grown on an MS medium with 2 mg of both IAA and kinetin. Then, a patho-system of Cicer arietinum calluses with Ascochyta rabiei was investigated, followed by a histological assessment of this interaction. The presence of the fruiting bodies of the pathogen was revealed in the calluses of the ‘ILC 32-97’ and ‘ILC 263’ genotypes. Notably, the latter showed a high sensitivity to the pathogen, as indicated by an abundance of pycnidia in its tissues. As for the ‘FLIP 84-92 C’ genotype, the histological sections showed a total absence of inter- and intracellular fruiting bodies of the pathogen in the callus tissues. Therefore, this genotype was considered as resistant to Ascochyta rabiei.
Metrics
References
Aasim M, Day S, Rezaei F, Hajizadeh M, Mahmud, ST, Ozcan S (2011). In vitro shoot regeneration from preconditioned explants of chickpea (Cicer arietinum L.) cv. Gokce. African Journal of Biotechnology 10(11):2020-2023. https://doi.org/10.5897/AJB10.823
Altaf N, Iqbal J, Salih Ahmad M. (1999). Tissue culture of Microsperma lentil (Lens culinaris mediki) cv. Massoor-85. Pakistan Journal of Botany 31(2):283-292.
Altınkut Uncuoğlu A, Bajrovic K, Gozukirmizi N (1997). Regeneration and hairy root formation of chickpea using callus derived plantlets and seedlings. International Chickpea and Pigenpea Newsletter 20:30-31.
Ameziane El Hassani A (1981). Modalités d’expression de la résistance d’un cultivar de pois chiche (Cicer arietinum L.) à Ascochyta rabiei (Pass.) Lab. PhD Thesis, ENSA Rennes, France.
Anwar F, Sharmila P, Pardha Saradhi P (2010). No more recalcitrant: Chickpea regeneration and genetic transformation. African Journal of Biotechnology 9(6):782-797. https://doi.org/10.5897/AJB2010.000-3009
Bharathi P, Elavarasi N (2012). Preliminary studies of reactor system designed for cell suspension culture of chickpea (Cicer aritenum). International Journal of Chemical Sciences and Applications 3(1):223-231.
Bouabdallah L (1986). Culture in vitro du melon (Cocumis melo L.) et tantative d’application à l’étude de la fusariose. PhD Thesis, Paris Sud (Orsay) Univ, France.
Boxus P, Bercetche J (1995). Multiplication végétative: micropropagation, embryogenèse somatique. Agence universitaire de la francophonie prod., Paris.
Casares A, Gonzalez A, Sánchez Tamés R (1994). Ultrastructure of calli from resistant and susceptible chestnut inoculated with Phytophthora cambivora. Journal of Physiopathology 142:19-26. https://doi.org/10.1111/j.1439-0434.1994.tb00003.x
Daayf F, El Bellaj M, El Hassni M, J’Aiti F, El Hadrami L (2003). Elicitation of soluble phenolics in date palm (Phoenix dactylifera) callus by Fusarium oxysporum f. sp. albedinis culture medium. Environmental and Experimental Botany 49:41-47. https://doi.org/10.1016/S0098-8472(02)00048-5
Dai GH, Andary C, Mandolot Cosson L, Boubals D (1995). Involvement of phenolic compounds in the resistance of grapevine callus to downy mildew (Plasmapara viticola). European Journal of Plant Pathology 101:541-547. https://doi.org/10.1007/BF01874479
DeZoeten GA, Gaad G, Haberlach GT, Helgeson JP (1982). Infection of Tabacco callus by Phytophthora parasitica var. nicotinae. Phytopthology 72(7):743-746. https://doi.org/10.1094/Phyto-72-743
Gallão MI, Cortelazzo AL, Fevereiro MPS, de Brito ES (2007). Response to chitin in suspension-cultured Citrus aurantium cells. Brazilian Journal of Plant Physiology 19(1)69-76. https://doi.org/10.1590/S1677-04202007000100008
Gautheret RJ (1959). La culture des tissus végétaux: techniques et réalisations. Masson, Paris.
Heiler R, Esnault R, Lance C (1995). Physiologie végétale. 2. Développement. Masson (5th ed), Paris.
Huda S, Siddique NA, Khatun N, Rahman MH, Morshed M (2003). Regeneration of shoot from cotyledon derived callus of chickpea (Cicer arietinum L.). Pakistan Journal of Biological Sciences 6(15):1310-1313. https://doi.org/10.3923/pjbs.2003.1310.1313
Ingram DS (1967). The expression of R-gene resistance to Phytophthora infestans in tissue cultures of Solanum tuberosum. Journal of general Microbiology 49:99-108. https://doi.org/10.1099/00221287-49-1-99
Ingram DS, Joachim I (1971). The growth of Peronospora farinosa f. sp. betae and sugar beet callus tissues in dual culture. Journal of General Microbiology 69:211-220. https://doi.org/10.1099/00221287-69-2-211
Jang JC, Tainter FH (1990). Cellular response of Pine callus to infection by Phytophtora cinnamomi. Phytopathology 80:1347-1352. https://doi.org/10.1094/Phyto-80-1347
Jones B, Ljiung K (2011). Auxin and cytokinin regulated each other’s levels via metabolic feedback loop. Plant Signalling & Behavior 6(6):901-904. https://doi.org/10.4161/psb.6.6.15323
Kavousi HR, Marashi H, Mazafari J, Bagheri AR (2009). Expression of phenylpropanoid pathway genes in chickpea defense against Race 3 of Ascochyta rabiei. Plant Pathology Journal 8(3):127-132. https://doi.org/10.3923/ppj.2009.127.132
Kebmann H, Barz W (1987). Accumulation of isoflavone and pterocarpans phytoalexin in cell suspension culture of different cultivars of chickpea (Cicer arietinum). Plant Cell Report 6(1):55-59. https://doi.org/10.1007/BF00269739
Khan S, Ahmad F, Ali F, Khan H, Khan A, Swati ZA (2011). Callus induction via different growth regulators from cotyledon explants of indigenous chick pea (Cicer arietinum L.) cultivars KK-1 and Hassan- 2K. African Journal of Biotechnology 10(40):7825-7830. https://doi.org/10.5897/AJB10.2378
Krause CR, Ichida JM, Shreider LR, Domir S (1996). Host parasite relationships to susceptible and resistant Elm callus cultures challenged with Ophiostoma ulmi (Buisman) Nannf. Journal of Environmental Horticulture 14(1):33-38. https://doi.org/10.24266/0738-2898-14.1.33
Kunwar, IK, Satyaprasad K., Ramarao P (1989). Histopathology of chickpea plants infected with Fusarium oxysporum f sp ciceri. International Chickpea Newsletter 20:17-18.
Lepoivre P (2003). Phytopathologie. De Boek (1st ed), Paris.
McComb JA, Hinch JM, Clarke AE (1987). Expression of field resistance in callus tissue inoculates with Phytophthora cinnamomi. Phytopathology 77:346-351. https://doi.org/10.1094/Phyto-77-346
Murashige T, Skoog F (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15(3):443-497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Naz S, Ali A, Ahmed Siddique F, Iqbal J (2008). Somatic embryogenesis from immature cotyledons and leaf calli of chickpea (Cicer arietinum L.). Pakistan Journal of Botany 48(2):523-531.
Neumann KH, Kumar A, Imani J (2009). Plant cell and tissue culture- A tool in biotechnology. Basis and application, principles and practice. Springer- Verlag (1st ed), Berlin.
Ochatt S J, Atif RM, Patat- Ochatt EM, Jacas L, Conreux C (2010). Competence versus recalcitrance for in vitro regeneration. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 38(2):102-108. https://doi.org/10.15835/nbha3824876
Sagare AP, Suhasini K, Krishnamurthy KV (1993). Plant regeneration via somatic embryogenesis in chickpea (Cicer arietinum L.). Plant Cell Reports 12:652-655. https://doi.org/10.1007/BF00232818
Sané D, Aberlanc- Bertossi F, Diatta LID, Guèye B, Daher A, Sagna M, Bogel A (2012). Influence of growth regulators on callogenesis and somatic embryo development in date palm (Phoenix dactylifera L.) sahalian cultivars. The Scientific World Journal 2012:1-8. https://doi.org/10.1100/2012/837395
Sani L, Mustapha Y (2010). Effect of genotype and 2, 4- d concentration on callogenesis in sugarcane (Saccharum spp. hybrids). Bayero Journal of Pure and Applied Sciences 3(1):238-240. https://doi.org/10.4314/bajopas.v3i1.58800
Savita V, Virk GS, Nagpal A (2010). Effect of explant type and different plant regulators on callus induction and plantlet regeneration Citrus jambhiri Lush. Environment and We: An International Journal of Science & Technology 5:97-106.
Singh M, Singh A (1990). Growth and sporulation of Neovossia indica in wheat callus culture. Indian Phytopathology 29:113-115.
Sultan IN, Yousafzai A, Sattar FA, Ahmmad F, Ibrahim SM, Hassanullah M, Arif B (2011). The effects of different concentrations and combinations of growth regulators on the callus formation of potato (Solanum tubrosum) explants. Current Research Journal of Biological Sciences 3(5):499-503.
Weidemann C, Tenhaken R, Höhl U, Barz W (1991). Medicarpin and Maackiain-3-O glucoside -6’-O-malonate conjugates are constitutive compounds in chickpea Cicer arietinum L. cell culture. Plant Cell Reports 10:371-374. https://doi.org/10.1007/BF00193162
Zaman MA, Manjur ABMK, Ahmed M, Islam MM (2010). Effect of 2,4-D on callus induction and subsequent morphogenesis in mature chickpea (Cicer arietinum L.) embryo culture. In: Islam AS, Haque MM, Sarker RH and Hoque MI (Eds). Proc. Sixth Intl. Plant Tissue Cult. & Biotech. Conf., Dhaka (Bangladesh) pp 53-58.
Zare MH, Bagheri AR, Zare MM (2011). Efficient protocol for break impasses of regeneration via callus for 20 genotypes of chickpea. International Journal of Plant Production 4(2):115-128. https://doi.org/10.22069/ijpp.2012.688
Zrÿd JP (1988). Cultures de cellules, tissus et organes végétaux. Fondements théoriques et utilisations pratiques. Presses polytechniques romandes, Laussane.
Downloads
Published
How to Cite
Issue
Section
License
Papers published in Notulae Scientia Biologicae are Open-Access, distributed under the terms and conditions of the Creative Commons Attribution License.
© Articles by the authors; licensee SMTCT, Cluj-Napoca, Romania. The journal allows the author(s) to hold the copyright/to retain publishing rights without restriction.
License:
Open Access Journal - the journal offers free, immediate, and unrestricted access to peer-reviewed research and scholarly work, due SMTCT supports to increase the visibility, accessibility and reputation of the researchers, regardless of geography and their budgets. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author.













.png)















