A comparative study between temporary immersion system and semi-solid cultures on shoot multiplication and plantlets production of two Moroccan date palm (Phoenix dactylifera L.) varieties in vitro
DOI:
https://doi.org/10.15835/nsb12210610Keywords:
bioreactor; liquid medium; micropropagation; organogenesis; shoot proliferationAbstract
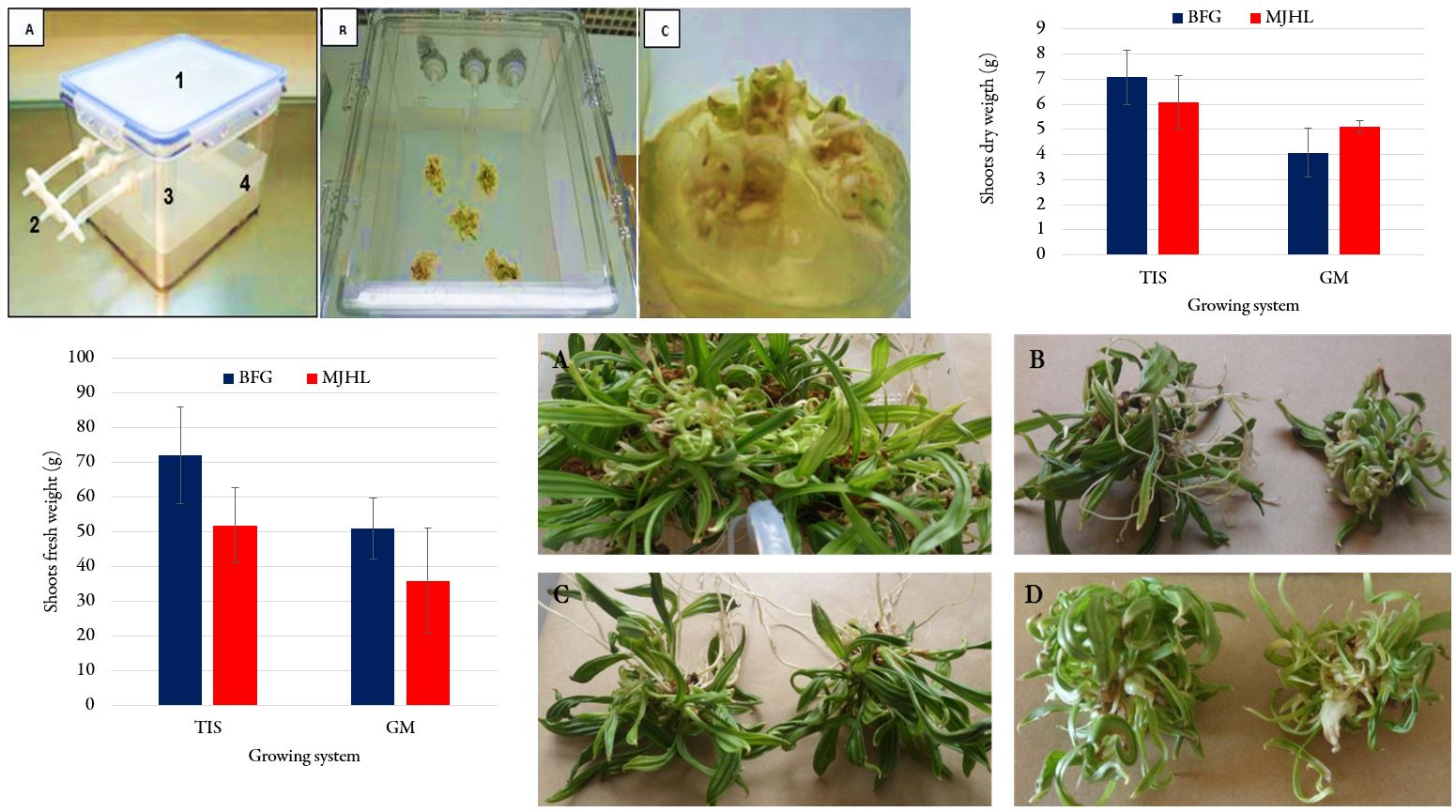
Date palm micropropagation is commonly performed on gelled media. However, it’s typically a labour-intensive system and consequently plantlets production cost is very high. Therefore, it is necessary to develop cost effective alternatives without compromising the quality of produced plant material. New technologies based on liquid media in bioreactors have been developed to reduce the handling time, while increasing the multiplication rates and plant quality. The present research focuses on the comparison between Temporary Immersion System (TIS) and gelled media (GM) culture systems of two Moroccan date palm varieties ‘Mejhool’ and ‘Boufeggous’. Obtained results indicated that shoot and root lengths as well as shoot fresh and dry weights were significantly (P < 0.05) higher in TIS compared to GM. Moreover, the vigour of obtained shoots was better in TIS compared to GM. Therefore, TIS-derived plantlets have shown an acclimatization rate of 95% while this rate for GM-derived plantlets was 82%. Hence, bioreactors, as a growing system based on TIS, can be a valid alternative to conventional systems for in vitro culture, resulting in a reduction of cost, shelving area requirements, labour and time for the mass propagation of date palm cultivars.
Metrics
References
Abahmane L (2017). Cultivar-dependent direct organogenesis of date palm from shoot tip explants. In: Al-Khayri JM, Jain SM, Johnson DV (Eds). Date palm biotechnology protocols, Methods in molecular biology. Humana Press, New York pp 3-15. https://doi.org/10.1007/978-1-4939-7156-5_1
Abahmane L (2011). Date palm micropropagation via organogenesis. In: Jain SM, Al-Khayri JM, Johnson DV (Eds). Date palm biotechnology. Springer, Dordrecht pp 69-90. https://doi.org/10.1007/978-94-007-1318-5_5
Akdemir HA, Süzerer V, Onay A, Tilkat E, Ersali Y, Çiftçi OY (2014). Micropropagation of the pistachio and its rootstocks by temporary immersion system. Plant Cell Tissue Organ Culture 117:65-76. https://doi.org/10.1007/s11240-013-0421-0
AlKhateeb AA, Alturki SM (2014). A comparison of liquid and semi-solid cultures on shoot multiplication and rooting of three date palm cultivars (Phoenix dactylifera L.) in vitro. Advances in Environmental Biology 8(16):263-269.
Al-Khayri JM, Naik PM (2017). Date palm micropropagation: Advances and applications. Ciência e Agrotecnologia 41(4):347-358. http://dx.doi.org/10.1590/1413-70542017414000217
Almusawi AHA, Sayegh AJ, Alshanaw AMS, Griffis JL (2017). Plantform bioreactor for mass micropropagation of date palm. In: Al-Khayri JM, Jain SM, Johnson DV (Eds). Date palm biotechnology protocols, Methods in molecular biology. Humana Press, New York pp 251-265. https://doi.org/10.1007/978-1-4939-7156-5_21
Aragón CE, Sánchez C, Gonzalez-Olmedo J, Escalona M, Carvalho L, Amâncio S (2014). Comparison of plantain plantlets propagated in temporary immersion bioreactors and gelled medium during in vitro growth and acclimatization. Biologia Plantarum 58(1):29-38. https://doi.org/10.1007/s10535-013-0381-6.
Arigundam U, Variyath AM, Siow YL, Marshall D, Debnath SC (2020). Liquid culture for efficient in vitro propagation of adventitious shoots in wild Vaccinium Vitis-idaea ssp. minus (lingonberry) using temporary immersion and stationary bioreactors. Scientia Horticulturae 264(5):1091-99. https://doi.org/10.1016/j.scienta.2020.109199
Benelli C, De Carlo A (2018). In vitro multiplication and growth improvement of Olea europaea L. cv. ‘Canino’ with temporary immersion system (Plantform™). 3 Biotech 8:317-321. https://doi.org/10.1007/s13205-018-1346-4
Carvalho LSO, Ozudogru EA, Lambardi M, Paiva LV (2019). Temporary immersion system for micropropagation of tree species: a bibliographic and systematic review. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 47(2):269-277. https://doi.org/10.15835/nbha47111305
Chakrabarty D, Hahn EJ, Yoon YJ, Paek KY (2003). Micropropagation of apple rootstock ‘M9 EMLA’ using bioreactor. Horticultural Science and Biotechnology 78(5):605-609. https://doi.org/10.1080/14620316.2003.11511671
De Klerk GJ, Ter Brugge J (2011). Micropropagation of dahlia in static liquid medium using slow-release tools of medium ingredients. Scientia Horticulturae 127:542-547.
Etienne H, Berthouly M (2002). Temporary immersion systems in plant micropropagation. Plant Cell, Tissue and Organ Culture 69:215-231. https://doi.org/10.1023/A:1015668610465
Farahani F, Majd A (2012). Comparison of liquid culture methods and effect of temporary immersion bioreactor on growth and multiplication of banana Musa, cv. ‘Dwarf Cavendish’. African Journal of Biotechnology 11(33):8302-8308.
Fki L, Bouaziz N, Kriaa W, Benjema-Masmoudi R, Gargouri-Bouzid R, Rival A, Drira N (2011). Multiple bud cultures of ‘Barhee’ date palm (Phoenix dactylifera L.) and physiological status of regenerated plants. Journal of Plant Physiology 168(14):1694-1700. https://doi.org/10.1016/j.jplph.2011.03.013
Gatti E, Sgarbi E, Ozudogru EA, Lambardi M (2017). The effect of PlantformTM bioreactor on micropropagation of Quercus robur in comparison to a conventional in vitro culture system on gelled medium and assessment of the microenvironment influence on leaf structure. Plant Biosystems 151(6):1129-1136.
https://doi.org/10.1080/11263504.2017.1340356
Georgieva L, Tsvetkov I, Georgieva M, Kondakova V (2016). New protocol for in vitro propagation of berry plants by TIS bioreactor. Bulgarian Journal of Agricultural Science 22(5):745-751.
Godoy S, Tapia E, Seit P, Andrade D, Sánchez E, Andrade P, Almeida AM, Prieto H (2017). Temporary immersion systems for the mass propagation of sweet cherry cultivars and cherry rootstocks: development of a micropropagation procedure and effect of culture conditions on plant quality. In vitro Cellular and Developmental Biology-Plant 53(5):494-504. https://doi.org/10.1007/s11627-017-9856-z
Ibraheem Y, Pinker I, Böhme M (2013). A comparative study between solid and liquid cultures relative to callus growth and somatic embryo formation in date palm (Phoenix dactylifera L.) cv. ‘Zaghlool’. Emirate Journal of Food and Agriculture 25(11):883-898. https://doi.org/10.9755/ejfa.v25i11.16661
Khierallah HSM, Bader SM (2007). Micropropagation of date palm (Phoenix dactylifera L.) cv. ‘Maktoom’ through direct organogenesis. Acta Horticulturae 736:213-224. https://doi.org/10.17660/ActaHortic.2007.736.19
Lyam PT, Musa ML, Jamaleddine ZO, Okere UA, Odofin WT, Carlos A (2012). The potential of temporary immersion bioreactors (TIBs) in meeting crop production demand in Nigeria. Journal of Biology and Life Science 3(1):66-86. https://doi.org/10.5296/jbls.v3i1.1156
Marbuna CL, Toruan-Mathiusa N, Utomoa RC, Liwanga T (2015). Micropropagation of embryogenic callus of oil palm (Elaeis guineensis Jacq.) using temporary immersion system. Procedia Chemistry 14:122-129.
McAlister B (2003). In vitro propagation of eucalyptus clones using a temporary immersion bioreactor system (RITA®). MSc Dissertation, University of Natal.
Murashige T, Skoog F (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiology Plantarum 15:473-97. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nagori R, Tathore P, Vyas S (2009). Liquid culture system stimulates in vitro growth and shoot multiplication in some important plant species of aravallis in Rajasthan. In: Kumar A, Shekhawat NS (Eds). Plant tissue culture and molecular markers. I.K. International Publishing House, New Delhi pp 395-405.
Othmani A, Bayoudh C, Sellemi A, Drira N (2017). Temporary immersion system for date palm micropropagation. In: Al-Khayri JM, Jain SM, Johnson DV (Eds). Date palm biotechnology protocols, Methods in molecular biology. Humana Press, New York pp 239-249. https://doi.org/10.1007/978-1-4939-7156-5_20
Othmani A, Mzid R, Bayoudh C, Trifi M, Drira N (2011). Bioreactors and automation in date palm micropropagation. In: SM Jain, Al-Khayri JM, Johnson DV (Eds). Date palm biotechnology. Springer, Dordrecht pp 119-136. https://doi.org/10.1007/978-94-007-1318-5_7
Othmani A, Bayoudh C, Drira N, Trifi M (2009). In vitro cloning of date palm (Phoenix dactylifera L.) cv. ‘Deglet Bey’ by using embryogenic suspension and temporary immersion bioreactor (TIB). Biotechnology & Biotechnological Equipment 23(2):1181-1188. https://doi.org/10.1080/13102818.2009.10817635
Persson J (2012). Evaluation of a new type of temporary immersion system (TIS) bioreactor for plant micropropagation. MSc Dissertation, Swedish University of Agricultural Sciences.
Rad MR, Zarghami R, Hassani H, Zakizadeh H (2015). Comparison of vegetative buds formation in two date palm cultivars, 'Medjool' and 'Mazafati' through direct organogenesis. International Journal of Farming and Allied Sciences 4(6):549-553.
Rajmohan K (2011). Date palm tissue culture: A pathway to rural development. In: SM Jain, Al-Khayri JM, Johnson DV (Eds). Date palm biotechnology. Springer, Dordrecht pp 29-45. https://doi.org/10.1007/978-94-007-1318-5_3
Ramli AB (2018). A low-cost temporary immersion bioreactor for micropropagation of local pineapple (Ananas Comosus L.). MSc Dissertation, Technology University of Malaysia.
Roels S, Noceda C, Escalona M, Sandoval J, Canal MJ, Rodriguez R, Debergh P (2006). The effect of headspace renewal in a temporary immersion bioreactor on plantain (Musa AAB) shoot proliferation and quality. Plant Cell Tissue Organ Culture 84:155-163. https://doi.org/10.1007/s11240-005-9013-y
Sandal I, Bhattacharya A, Ahuja PS (2001). An efficient liquid culture system for tea shoot proliferation. Plant Cell Tissue Organ Culture 65:75-80. https://doi.org/10.1023/A:1010662306067
Schönherr J (2006). Characterization of aqueous pores in plant cuticles and permeation of ionic solutes. Journal of Experimental Botany 57(11):2471-2491. https://doi.org/10.1093/jxb/erj217
Shandil AS, Tuia VS (2015). Micropropagation of breadfruit (A. altilis) enhanced using a bioreactor system. Acta Horticulturae 1101:159-163. http://dx.doi.org/10.17660/ActaHortic.2015.1101.24
Vyas S, Rao SM, Suthar RK, Purohit SD (2008). Liquid culture system stimulates in vitro growth and shoot multiplication in four medicinally important plants. Medicinal and Aromatic Plant Science and Biotechnology 2(2):96-100.
Welander M, Persson J, Asp H, Zhu LH (2014). Evaluation of a new vessel system based on temporary immersion system for micropropagation. Scientia Horticulturae 179:227-232. http://dx.doi.org/10.1016/j.scienta.2014.09.035
Yan H, Liang C, Li Y (2010). Improved growth and quality of Siraitia grosvenorii plantlets using a temporary immersion system. Plant Cell Tissue Organ Culture 103:131-135. https://doi.org/10.1007/s11240-010-9752-2
Yang SH, Yeh DM (2008). In vitro leaf anatomy, ex vitro photosynthetic behaviour and growth of Calathea orbifolia (Linden) Kennedy plants obtained from semi-solid medium and temporary immersion systems. Plant Cell Tissue & Organ Culture 93:201-207. https://doi.org/10.1007/s11240-008-9363-3
Downloads
Published
How to Cite
Issue
Section
License
Papers published in Notulae Scientia Biologicae are Open-Access, distributed under the terms and conditions of the Creative Commons Attribution License.
© Articles by the authors; licensee SMTCT, Cluj-Napoca, Romania. The journal allows the author(s) to hold the copyright/to retain publishing rights without restriction.
License:
Open Access Journal - the journal offers free, immediate, and unrestricted access to peer-reviewed research and scholarly work, due SMTCT supports to increase the visibility, accessibility and reputation of the researchers, regardless of geography and their budgets. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author.













.png)















